International Journal of Cancer and Clinical Research
Cancer Antigen 125 Levels can be Used as a Tumor Marker for Monitoring Patients with Endometrial Serous Carcinoma?
M W Hegazy1,2*, Wael H Elsawy2, A Tolba3 and M Shoukri4
1Department of Radiation Oncology, King Faisal Specialist Hospital & Research Center, Saudi Arabia
2Department of Clinical Oncology & Nuclear Medicine, Zagazig Faculty of Medicine, Egypt
3Department of pathology, King Faisal Specialist Hospital & Research Center, Saudi Arabia
4Department of Cell biology & Biotechnology, King Faisal Specialist Hospital & Research Center, Saudi Arabia
*Corresponding author:
Mohamed W Hegazy, Department of Radiation Oncology, King Faisal Specialist Hospital & Research Center, Kingdom of Saudi Arabia, PO Box 3354, Riyadh 11211 MBC 64, Saudi Arabia, Tel: 966550742859, E-mail: mhegazy@kfshrc.edu.sa
Int J Cancer Clin Res, IJCCR-3-075, (Volume 3, Issue 6), Research Article; ISSN: 2378-3419
Received: October 31, 2016 | Accepted: November 28, 2016 | Published: December 01, 2016
Citation: Hegazy MW, Elsawy WH, Tolba A, Shoukri M (2016) Cancer Antigen 125 Levels can be Used as a Tumor Marker for Monitoring Patients with Endometrial Serous Carcinoma?. Int J Cancer Clin Res 3:075. 10.23937/2378-3419/3/6/1075
Copyright: © 2016 Hegazy MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To determine the levels of CA-125 preoperative, postoperative and during follow up of patients with uterine serous adenocarcinoma (USAC) and evaluate its role as a tumor marker or not in these patients by its level changes on staging, cure and recurrence.
Methods: Database was reviewed for all USAC patients. Thirty three patients who were treated and had serial CA-125 (normal level up to 35 U/ml) from 2007 to 2014 at our institution were included in this study.
Results: With mean follow up of 26 months, there are 7 patients with initial normal CA-125 with only intrauterine disease and 26 patients with initial high CA-125 with extrauterine disease (p value 0.024). Mean follow up of early stage was 42 months while late stage was 20 months (p 0.019).With doubling levels of CA-125, there was a statistical significant effect on follow up period (p 0.027). In early stage, 3 out of 9 patients had recurrence; while 13 out of 17 patients in advanced stage, post therapy after normalization of CA-125 (p = 0.04), all recurrent cases had high CA-125 (p < 0.001).
Conclusion: Our study with these findings showed that preoperative and serial levels of serum CA-125 correlate with disease stage, follow up period and may be having clinical significant and preceding disease recurrence and /or progression.
Keywords
CA125, Endometrial serous carcinoma
Background
Uterine cancer is the most common malignancy of the female genital tract in the United States [1], a situation which is similar at our institution [2].
Endometrial adenocarcinoma is the most common type of uterine cancer. Endometrial cancer is classified into two subtypes (I and II), which reflect general characteristics of its clinicopathological spectrum. Uterine serous adenocarcinoma (USAC) is under Type II neoplasms which are associated with more aggressive behavior than type I tumors. While they comprise 10-20% of endometrial carcinomas, they account for 40% of deaths from the disease [3-5].
USAC may develop from endometrial intraepithelial carcinoma (EIC), a lesion related to malignant transformation of the endometrial surface epithelium, against a background of endometrial atrophy [6,7]. However, EIC was commonly found in association with extrauterine serous carcinoma, with both sites having identical clones of p53 mutations [8]. This finding suggests that EIC represents an early form of USAC rather than its true precursor [9]. A relatively new entity, "endometrial glandular dysplasia", which histologically bridges benign endometrium and EIC, may be the putative precursor lesion to USC [10].
USAC is a more aggressive histologic variant of malignant epithelial tumors, with a higher incidence of extrauterine disease at presentation [11-13]. Serous adenocarcinoma is considered high grade by default, although it is staged using the same FIGO/AJCC staging system as endometrial cancers [14]. Both the NCCN panel and the SGO recommended that CA-125 and MRI/CT may be useful before surgery to assess if extrauterine disease is present; PET may also be useful. Patterns of failure often mimic those of ovarian cancer [11]. Multimodality therapy is typically recommended for this tumors [15,16]. Optimal cytoreduction and adjuvant platinum/taxane-based chemotherapy appear to improve survival, while adjuvant radiotherapy may contribute to loco-regional disease control [11].
The 5-year survival rate for all stages of USAC is only 53% compared with 83% for endometrioid carcinoma [17]. Several studies have shown a correlation between preoperative levels of CA-125 and presence of extrauterine disease at the time of surgery as well as advanced stage and survival in women with USAC [18-21]. Serum CA-125 levels in USAC have been assessed in many previous studies [19-26] and their surveillance may be a useful indicator of disease response or progression [19,26]. The aim of our study was to evaluate the clinical significance of rising CA-125 levels in patients with USAC.
Methods
The medical records of women diagnosed as USAC, had regular CA-125 levels and treated between 2007 till end of 2013 at king Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia were reviewed. Thirty three patients with histologically proven diagnosis of USAC were identified and selected for analysis. Records were reviewed for age, stage of disease, first and serial CA-125 readings (post-surgery, post chemotherapy and during follow up period). Twenty seven patients underwent comprehensive surgical staging and cytoreduction and received adjuvant treatment at our institution. Nine patients out of thirty three had second cancers (four breasts, two papillary thyroids, one skin, one hepatoma and one Non-Hodgkin's lymphoma). The normal range of CA-125 is ≤ 35 U/mL.
Statistical Analysis
Chi square test was used to test independence between two categorical variables. Differences in median were tested using Mann-Whitney two samples test. When we have three groups or more, median test was done using Kruskal-Wallis test. Correlation between variables was assessed using Spearman's Rank test. Type one error rate of 5% is used throughout.
Results
With a mean follow up period of 26 months [3-16], we had 33 patients of USAC. Table 1 illustrates their characteristics. Patients were under regular follow up by clinical, radiological and serial CA-125 level. The mean age was 69 years; two patients (6%) were treated by surgery alone, six patients (18%) were inoperable and/or unfit for surgery, four patients (12%) were unfit for chemotherapy due to poor performance status; four patients (12%) were treated by chemotherapy alone, twenty four patients (72.7%) received adjuvant radiation therapy. Stage 4 was the commonest group and was in 16 patients (48.5%), while stage 2 was the least group and was in 3 patients (9%). We merged stages 2 and 1 due to small number of patients. Increase in initial CA-125 was in 26 patients (78.8%), where one patient (3%) stage 1, two patients (6.1%) were stage 2, seven patients (21.2%) were stage 3 and sixteen patients (48.5%) were stage 4, p = 0.23 so Initial high CA-125 is correlated with advanced stages (3 & 4) (p = 0.012) and extra uterine disease (p = 0.024). The mean follow up for early stage (1,2) was 42 months, while advanced stage (3,4) was 20 months (p = 0.019).When we divided the patients to three groups according to initial CA-125 level( ≤ 35, 36-70 & > 70U/mL), the mean follow up time is decreased (54, 24 &15 months), p = 0.027. The mean CA-125 regarding to disease stage is 24, 46, 56 & 905 (p = 0.249). Only 33.3% of early stage has initial high CA-125; while 96% of advanced stage has initial high CA-125 (p = 0.001). In early stage, 3 patients (30%) had recurrence (2 of them had initial normal CA-125) post therapy after normalization of CA-125; while in advanced stage, 13 patients (54%) had recurrence post therapy after normalization of CA-125 (p = 0.04) and seven patients (29%) had stable and/or progressive disease (p = 0.012), meanwhile patients with stage III/IV disease were more likely to recur or progress when compared to patients with stage I/II disease. All recurrent cases had high CA-125 (p < 0.001), table 2.
![]()
Table 1: Patients characteristics.
View Table 1
![]()
Table 2: Patients stages, marker level and treatment outcome relationship.
View Table 2
Discussion
In our study we tried to evaluate the potential role of CA-125 as a serum tumor marker for this uncommon high-risk endometrial cancer either in initial staging, post treatment response, disease recurrence or progression.
We found CA-125 was beneficial in three scenarios. Firstly, at diagnosis CA-125 correlated with early intrauterine and advanced extrauterine stages, secondly with disease remission clinically and radiologically post treatment showed normalized CA-125, and lastly increasing in cases of recurrence and/or progression during follow up period as shown in figure 1.
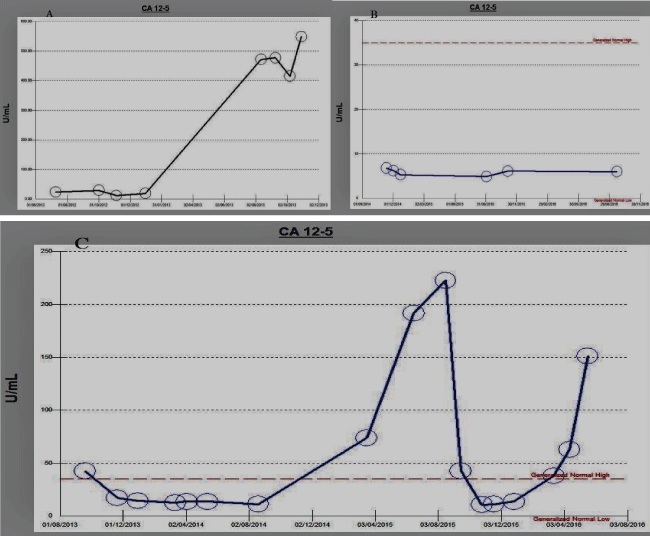
.
Figure 1: Three different patients A) displayed patient stage 1with normal follow up CA-125 then with recurrence started increasing; B) displayed patient with stage 3 with cure and normalized CA-125 during the whole follow up period; C) displayed patient with stage 4 cured and showed normal CA-125then multiple recurrences showed high CA-125 levels.
View Figure 1
No previously published studies have clearly answered the question of the role of CA-125 in USAC as shown below.
Niloff, et al. reported that CA-125 was elevated in 78% of cases with stage IV or recurrent disease [18]. Others also reported a correlation between initial high CA-125 and advanced disease at the time of surgery [27-29]. Price, et al reported that CA-125 may reflect disease stage but not disease status during follow up period [23]. Abramovich, et al reported that 73% of cases with high CA-125 correlated with clinical relapse [18]. Moller, et al. reported that initial CA-125 was not predictive for the extent of cytoreduction surgery for those cases [30]. Olawaiye, et al. and Gupta, et al. concluded that CA-125 is a useful prognostic marker, as preoperative values that correlate with stage, and tumor burden, similar to our study [20,21]. Frimer, et al. reported that rising CA-125 may be clinically significant and precedes disease recurrence, and due to lack of standard biomarkers, they suggest a potential role for CA-125 to be as a monitor of surveillance for those patients in clinical remission, similar to our results [26]. Boruta, et al. have published a literature review about USAC, concluded that unlikely there have been conflicting results regarding to CA-125 utility, in spite of increasing CA-125 more than normal is correlated with increasing the risk of cancer related death however; CA-125 cannot be used as surrogate marker for disease status [11].
Our results are preliminary report so we have limitations like it is a retrospective review, a single institutional experience and small number of patients that may be corrected by a prospective, large, randomized multi-institutional cohort.
Conclusion
Our study with these findings showed that preoperative and serial levels of serum CA-125 correlate with disease stage, follow up period and may be having clinical significant and preceding disease recurrence and/or progression.
Compliance with Ethical Standards
This study has been approved by the institutional ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to therapy; however, for retrospective review of data with less than minimal risk to the patients, no consent was required by the ethics committee. All authors declare that there is no conflict of interest.
References
-
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics 64: 9-29.
-
(2013) KFSH&RC, Tumor Registry, Annual Report: 6.
-
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15: 10-17.
-
Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, et al. (2010) Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 21: 1851-1856.
-
Moore KN, Fader AN (2011) Uterine papillary serous carcinoma. Clin Obstet Gynecol 54: 278-291.
-
Sherman ME, Bitterman P, Rosenshein NB, Delgado G, Kurman RJ (1992) Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic features. Am J Surg Pathol 16: 600-601.
-
Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ (1995) Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol 26: 1260-1267.
-
Zheng W, Schwartz PE (2005) Serous EIC as an early form of uterine papillary serous carcinoma: recent progress in understanding its pathogenesis and current opinions regarding pathologic and clinical management. Gynecol Oncol 96: 579-582.
-
Cheng L, Song SY, Pretlow TG, Abdul-Karim FW, Kung HJ, et al. (1998) Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst 90: 233-237.
-
Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, et al. (2004) Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. Int J Surg Pathol 12: 207-223.
-
Boruta DM, Gehrig PA, Fader AN, Olawaiye AB (2009) Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol 115: 142-153.
-
Cirisano FD, Robboy SJ, Dodge RK, Bentley RC, Krigman H, et al. (2000) The outcome of stage I-II clinically and surgically staged papillary serous and clear cell endometrial cancers when compared with endometrioid carcinoma. Gynecol Oncol 77: 55-65.
-
Hendrickson MR, Longacre TA, Kempson RL (1994) Uterine papillary serous carcinoma revisited. Gynecol Oncol 54: 261-263.
-
Edge SB, Byrd DR, Compton CC (2010) AJCC Cancer Staging Manual (7th edn), Springer, New York, USA.
-
Goldberg H, Miller RC, Abdah-Bortnyak R, Steiner M, Yildiz F, et al. (2008) Outcome after combined modality treatment for uterine papillary serous carcinoma: a study by the Rare Cancer Network (RCN). Gynecol Oncol 108: 298-305.
-
Grice J, Ek M, Greer B, Koh WJ, Muntz HG, et al. (1998) Uterine papillary serous carcinoma: evaluation of long-term survival in surgically staged patients. Gynecol Oncol 69: 69-73.
-
Mendivil A, Schuler KM, Gehrig PA (2009) Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control 16: 46-52.
-
Niloff JM, Klug TL, Schaetzl E, Zurawski VR, Knapp RC, et al. (1984) Elevation of serum CA125 in carcinomas of the fallopian tube, endometrium, and endocervix. Am J Obstet Gynecol 148: 1057-1058.
-
Abramovich D, Markman M, Kennedy A, Webster K, Belinson J Serum (1999) CA-125 as a marker of disease activity in uterine papillary serous carcinoma. J Cancer Res Clin Oncol 125: 697-698.
-
Olawaiye AB, Rauh-Hain JA, Withiam-Leitch M, Rueda B, Goodman A (2008) Utility of pre-operative serum CA-125 in the management of uterine papillary serous carcinoma. Gynecol Oncol 110: 293-298.
-
Gupta D, Gunter MJ, Yang K, Lee S, Zuckerwise L, et al. (2011) Performance of serum CA125 as a prognostic biomarker in patients with uterine papillary serous carcinoma. Int J Gynecol Cancer 21: 529-534.
-
Tseng PC, Sprance HE, Carcangiu ML, Chambers JT, Schwartz PE (1989) CA 125, CA 125, NB/70K, and lipid-associated sialic acid in monitoring uterine papillary serous carcinoma. Obstet Gynecol 74: 384-387.
-
Price FV, Chambers SK, Carcangiu ML, Kohorn EI, Schwartz PE, et al. (1998) CA 125 may not reflect disease status in patients with uterine serous carcinoma. Cancer 82: 1720-1725.
-
Hoskins PJ, Le N, Correa R (2011) CA 125 normalization with chemotherapy is independently predictive of survival in advanced endometrial cancer. Gynecol Oncol 120: 52-55.
-
Roelofsen T, Mingels M, Hendriks JC, Samlal RA, Snijders MP, et al. (2012) Preoperative CA-125 predicts extra-uterine disease and survival in uterine papillary serous carcinoma patients. Int J Biol Markers 27: 263-271.
-
Frimer M, Hou JY, McAndrew TC, Goldberg GL, Shahabi S (2013) The clinical relevance of rising CA-125 levels within the normal range in patients with uterine papillary serous cancer. Reproductive Sciences 20: 449-455.
-
Duk JM, Aalders JG, Fleuren GJ, de Bruijn HW (1986) CA 125: a useful marker in endometrial carcinoma. Am J Obstet Gynecol 155: 1097-1102.
-
Patsner B, Mann WJ, Cohen H, Loesch M (1988) Predictive value of preoperative serum CA 125 levels in clinically localized and advanced endometrial carcinoma. Am J Obstet Gynecol 158: 399-402.
-
Sood AK, Buller RE, Burger RA, Dawson JD, Sorosky JI (1997) Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obstet Gynecol 90: 441-447.
-
Moller KA, Gehrig PA, Van Le L, Secord AA, Schorge J (2004) The role of optimal debulking in advanced stage serous carcinoma of the uterus 94: 170-174.





