International Journal of Cancer and Clinical Research
Three-Dimensional Ultrasound: Is it Useful for Decision Making in the Management of Rectal Cancer? Is 3D Ultrasound Useful in Rectal Tumor?
Sthela M Murad-Regadas1*, Romulo M Almeida2, Rosilma G Lima Barreto3, Doryane MR Lima4, Francisco Sergio Pinheiro Regadas1, Lusmar Veras Rodrigues1, Francisco Coracy C Monteiro1, Paulo G Oliveira2, João Batista Barreto3 and Univaldo Etsuo Sagae4
1Department of Surgery, Federal University of Ceará, Brazil
2Department of Surgery, Federal University of Brasilia, Brazil
3Department of Surgery, Federal University of Maranhao, Brazil
4Colorectal Surgery, Gastroclinica Cascavel, Brazil
*Corresponding author:
Sthela M Murad-Regadas, Associate Professor, Department of Surgery, Coordenator of Unit of Pelvic Floor and Anorectal Physiology, School of Medicine, Clinical Hospital, Federal University of Ceará; Department of Colorectal Surgery San Carlos Hospital, Fortaleza-CE, Av Pontes Vieira, 2551, Fortaleza, Ceará, 60130-241 Brazil, E-mail: smregadas@hospitalsaocarlos.com.br
Int J Cancer Clin Res, IJCCR-3-068, (Volume 3, Issue 5), Original Article; ISSN: 2378-3419
Received: September 01, 2016 | Accepted: October 14, 2016 | Published: October 17, 2016
Citation: Murad-Regadas SM, Almeida RM, Barreto RGL, Lima DMR, Regadas FSP, et al. (2016) Three-Dimensional Ultrasound: Is it Useful for Decision Making in the Management of Rectal Cancer? Is 3D Ultrasound Useful in Rectal Tumor? Int J Cancer Clin Res 3:068. 10.23937/2378-3419/3/5/1068
Copyright: © 2016 Murad-Regadas SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The three-dimensional ultrasound (3D-US) has been used to evaluate tumor response and downsizing after chemoradiation (CRT), and to determine whether there is residual tumor or a complete response.
Objective: To determine the usefulness of this 3D-US protocol in pretreatment staging of rectal cancer and in evaluating tumor response downsizing after CRT in making treatment decisions.
Methods: Consecutive patients treated for rectal cancer or adenomas in 4 colorectal centers in Brazil were included. Patients with early-stage rectal cancer identified by 3D-US were assigned to receive resection only, and those with a later stage were assigned to receive CRT. A second 3D-US was performed after CRT to detect the presence of residual tumor and metastatic lymph nodes or complete response. Sphincter-preserving surgery was performed in patient with a distance ≥ 1.5 cm. Agreement of pathologic analysis with post-CRT 3D-US regarding rectal wall infiltration and lymph node involvement was evaluated.
Results: Overall agreement regarding tumor status was classed as almost perfect (κ = 0.89) in patients who underwent resection without CRT. For post-CRT 3D-US, overall agreement regarding identification of residual tumor and complete response was substantial (κ = 0.62); sensitivity, 98%; specificity, 65%; positive predictive value, 87%; and negative predictive value, 94%. Overall agreement regarding lymph node involvement was moderate (κ = 0.42); sensitivity, 46%; specificity, 94%.
Conclusions: Rectal cancer was accurately staged and managed using 3D-US. After CRT, 3D-US showed high sensitivity in identifying residual tumor in the rectal wall or complete response and ability to quantify residual tumor. The most important parameter for the selection of patients for sphincter preservation was the distance between the tumor and the sphincter. Safe distal margins should be at least 1.5 cm. 3D-US was a reproducible method for evaluating patients with rectal cancer and enabled following an established protocol with good results.
Keywords
Rectal neoplasm, Endoscopic ultrasound, Endorectal ultrasonography, Decision making
Introduction
Endorectal ultrasonography is a useful method for local staging of rectal cancer, and several studies have shown high accuracy for assessing local depth of invasion of rectal carcinoma [1-4], although accuracy in the assessment of lymph node metastases varies widely [2-7]. In the evaluation of the response after neoadjuvant chemoradiotherapy in rectal cancer, difficulties in diagnosing the degree of downstaging decrease accuracy of re-staging the tumor [8-11]. Recent advances in technology and development of anorectal three-dimensional ultrasound (3D-US) make it possible to evaluate high-resolution images in multiple planes and allow review of the volumetric image in real time [12]. This method has been used to evaluate tumor response and downsizing after CRT, and to determine whether there is residual tumor or a complete response [12,13]. Murad-Regadas, et al. [13] evaluated criteria for 3D-US assessment of tumor response and developed a protocol for measurements that may help in choosing the appropriate treatment after CRT. So, the aim of this study was to determine the usefulness of this 3D-US protocol in pretreatment staging of rectal cancer and in evaluating tumor response downsizing after chemoradiation (CRT) in making treatment decisions, it is necessary to validate it and investigate whether the results can be reproduced. We therefore conducted a multicenter study using this protocol and compared the results of 3D-US with findings of standard pathologic analysis of resection specimens.
Materials and Methods
Patients
Consecutive patients treated for rectal cancer or adenomas between January-2010 and October-2014 at 4 tertiary-care colorectal centers in Brazil were prospectively included in the study. Patients who did not complete the all steps of this study or who did not accept to participate were excluded.
Approval was obtained from the Research Ethics Committees of the participating centers prior to initiation of the trial, and all patients provided written informed consent.
Study design
Patients were initially evaluated clinically, followed by colonoscopy and 3D-US. Patients considered to have early-stage rectal cancer were assigned to receive resection only, and those with a later stage were assigned to receive neoadjuvant CRT as well as underwent distant staging using methods like a torax, abdominal and pelvic tomography computed. Those who underwent neoadjuvant CRT had a second 3D-US performed by the same ultrasonographer 50-60 days after CRT to detect the presence of residual tumor and metastatic lymph nodes or complete response. The decision whether to perform sphincter-preserving surgery or abdominoperineal resection was based on the 3D-US measurements. The 3D-US was performed by 4 examiners, 1 from each of the 4 Brazilian colorectal centers, and all examiners involved in the study had previous experience with anorectal 3D-US and the measurements were standardized as far as possible to allow comparison. A team of colorectal surgeons from the 4 colorectal centers who specialized in coloproctology (certified by the Brazilian Board of Colorectal Surgery) performed all operations.
3D-US technique
Scanning was performed with a 3D-US endoprobe (Pro-Focus 2052; 16 MHz; focal distance 4.8-6.2 cm, BK Medical, Herlev- Denmark). Images up to 6.0 cm long were captured along the proximal-distal axis for up to 55 seconds by moving 2 crystals (axial and longitudinal) on the extremity of the transducer. The examination involved a series of transaxial microsections up to 0.20 mm thick, producing a high-resolution digitalized volumetric image. Volume was displayed as a 3D cube image and recorded and analyzed in multiple planes. Patients were examined in the left lateral position after rectal enema (completed 2 hours prior to scanning).
After digital rectal examination, a rigid proctoscope was inserted into the rectum past the level of the tumor. The endoprobe was introduced within the proctoscope and filled with degassed water, providing an acoustic interface. Three scans were performed to evaluate: a) the proximal perirectal fat and adjacent to the tumor; b) tumor size; and c) the relationship between the tumor and the anal canal sphincter muscles.
Evaluation of pretreatment ultrasound
Rectal wall infiltration and metastatic lymph nodes in the perirectal fat were evaluated according to the criteria of Hildebrandt and Feifel [14] (uTuN). Hypoechoic (or tumor-like) areas with irregular borders were considered to be metastatic lymph nodes. In contrast, oval structures with regular borders and a central hyperechoic area (corresponding to the hilum) were suggestive of inflammatory nodes [15]. The examination included the measurement of tumor length in cm (distance from the proximal to the distal edge of the tumor), anal canal invasion, and the distance between the distal tumor edge and the proximal edge of the anal canal muscle, corresponding to the puborectalis muscles and internal anal sphincter (IAS) in the posterior quadrant.
Early-stage rectal cancer
Patients with cancer staged as uT0, uT1N0 or uT2N0 were considered to have early-stage rectal cancer and were assigned to receive resection only. Agreement between preoperative 3D-US results and postoperative pathologic analysis regarding rectal wall infiltration (uT) was evaluated in all patients in this group, regardless of type of surgery. Agreement regarding identification of metastatic lymph nodes (uN) in the perirectal fat was evaluated only in patients who underwent radical surgery.
Later-stage rectal cancer
Neoadjuvant CRT: Patients with cancer stage uT2N0 with anal canal invasion or close it (less than 1 cm) uT2N1, or with uT3 or uT4/uN0 or uN1 were assigned to receive long-course neoadjuvant CRT using conventional doses of 1.8 to 2 Gy per fraction over 5 to 6 weeks, for a total dose of 45 to 50.4 Gy [16,17]. The protocol for continuous-infusion chemotherapy was 5-fluorouracil (425 mg/m2/day) with leucovorin (20 mg/m2/day) for 6 weeks.
Post CRT evaluation: In patients who underwent CRT, the initial clinical response was evaluated 50 to 60 days after completion of the CRT. A complete clinical response was defined as the absence of residual ulceration, mass, or significant rectal wall irregularity, as previously described [18].
A second 3D-US scan was also performed 50 to 60 days after completion of the CRT. Residual rectal cancer was not restaged (uT) following CRT [12,13]. Instead, the response was classified as residual tumor or complete response (total tumor regression). Assessments of tumor length (L), length reduction ratio (LR), and distance (D) between the distal tumor edge and the proximal border of the sphincter (cm) or anal canal invasion were repeated. The tumor size reduction ratio was calculated as a percentage [(pretreatment-posttreatment length)/pretreatment length] × 100. The perirectal fat was also reevaluated to identify residual metastatic lymph nodes.
Residual tumor was characterized on 3D-US by heterogeneous images with hyperechoic areas due to residual tumor associated with hypoechoic areas caused by the inflammatory process or images that were similar to pre-CRT images but more hypoechoic due to inflammation. Rectal wall layers were distinguishable at the former tumor location. The 3D-US findings for complete response (total lesion regression) were characterized by rectal wall layers and/or sphincter muscles that were clearly identified at the former tumor location and images that suggested fibrosis corresponding to thickening of the rectal wall, or hypoechoic images that made it possible to distinguish the layers in the area occupied by the tumor. The perirectal fat was also reevaluated to visualize there is no residual metastatic lymph nodes.
Treatment after CRT: If post-CRT 3D-US showed residual tumor invading the anal canal or a distance of < 1.5 cm between the distal tumor edge and the proximal border of the IAS, abdominoperineal resection with total mesorectal excision was performed. Sphincter-saving surgery with total mesorectal excision was performed if post-CRT 3D-US showed a complete response to CRT or a distance of ≥ 1.5 cm between the distal tumor edge and the proximal border of the IAS.
More recently, one center adopted a watch-and-wait approach for patients with both a complete clinical and a complete ultrasound response after CRT. These patients were offered no immediate surgery and were enrolled in a strict follow-up program including monthly follow-up visits with reassessment of tumor by clinical and 3D-US examinations.
Assessments: Tumor length, length reduction ratio, and distance between the distal tumor edge and the proximal border of the sphincter were measured in the post-CRT treatment groups (abdominoperineal resection and sphincter-saving surgery). Agreement between the results of post-CRT 3D-US and postoperative pathologic findings was examined regarding identification of complete response, residual tumors in the rectal wall, and lymph node involvement.
Follow-up: Patients with the watch-and-wait approach were followed at clinic visits every 1-2 months for the first year, every 3 months during the second year, and every 6 months during the third year and thereafter. Patients who underwent surgery were followed every 6 months for the first 2 years and annually thereafter.
Statistical analysis: Student's t test was used to compare tumor length, length reduction ratio, and distance between the distal tumor edge and the proximal border of the sphincter or anal canal invasion in patients who underwent abdominoperineal resection vs those who underwent sphincter-saving surgery.
In patients who underwent surgery without CRT, agreement between preoperative 3D-US and pathologic findings was evaluated regarding stage in the rectal wall and/or lymph nodes. In patients who received neoadjuvant CRT, agreement between post-CRT 3D-US and pathologic findings was evaluated regarding the identification of complete response or residual tumors in the rectal wall and/or lymph nodes. Agreement was estimated with the kappa (κ) statistic (< 0 = no agreement; 0-0.19 = poor agreement; 0.20-0.39 = fair agreement; 0.40-0.59 = moderate agreement; 0.60-0.79 = substantial agreement; 0.80-1.00 = almost perfect agreement) [19]. Sensitivity, specificity, and positive and negative predictive values were calculated. The level of statistical significance was set at p < 0.05.
Data were analyzed using SPSS software (version 14.0 for Windows; IBM-SPSS, Chicago, IL).
Results
A total of 94 patients participated, including 61 (65%) women and 33 (35%) men. The median age was 59 (range, 28-79) years. The tumor recurred in 6 (6%) patients. A total of 5 (5%) patients died: 2 of recurrent disease, 1 of postoperative complications, and 2 of other causes. The median follow-up duration for the surviving patients was 2 (range, 0.25-6) years.
Resection only
Based on pretreatment evaluation of cancer stage with 3D-US, the following patients were assigned to undergo resection without neoadjuvant CRT: 12 patients with uT0N0, 3 patients with uT1N0, and 1 patient with uT2N0. In addition, 2 patients with uT3 tumors (1 with N1) underwent only resection without neoadjuvant CRT because they had diabetic and cardiac comorbidity. Thus, a total of 18 patients underwent resection without neoadjuvant CRT, including 11 (61%) women and 7 (39%) men. The median age was 54 (range, 28-73) years.
The mean tumor length was 2.9 ± 1.0 (range, 1.3-4.7) cm and the mean width was 1.5 ± 0.24 (range, 0.5-2.7) cm. The mean distance between the distal tumor edge and the proximal border of the sphincter was 2.2 ± 1.7 (range, 0.3-7) cm. The type of procedure was endoscopic resection in 5 (28%) patients (uT0N0) and surgical resection in 13 (72%) patients. Of the patients with surgical resection, 4 had local excision (uT0N0), 5 had transanal endoscopic microsurgery (3 uT0N0 and 2 uT1N0), and 4 had radical surgery (low anterior resection with sphincter preservation). Of them, 2 patients with uT3 tumor, one uT2N0 and one uT1N0 had extension lesion (Figure 1 and Figure 2). No patients had recurrence and no deaths were reported.
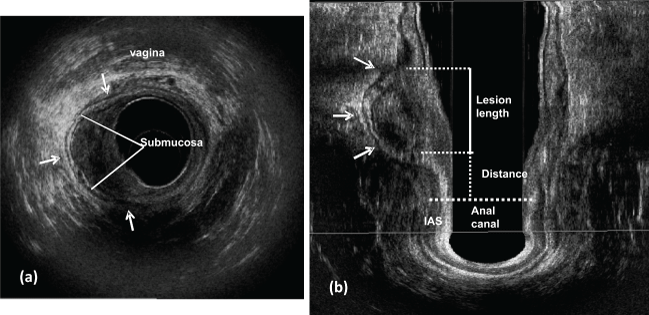
.
Figure 1: Polyp is located in the right lateral posterior rectal wall (arrows). uT0 - thickened muscularis - mucosa layer and submucosa is intact (line).
a) Axial plane; b) coronal plane - lesion length and the distance between the distal tumor edge and the proximal border of the muscles. IAS: internal anal sphincter.
View Figure 1
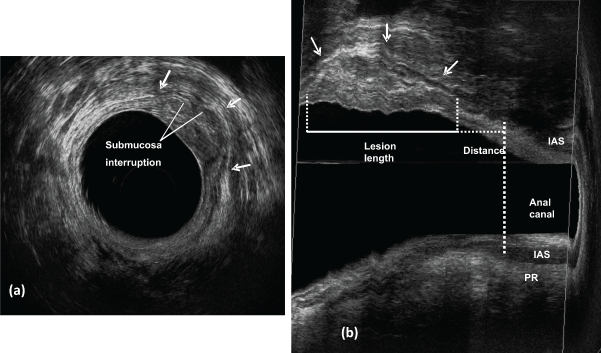
.
Figure 2: Early invasive cancer - uT1 - the middle hyperechoic layer corresponding to submucosa is interrupted and the hypoechoic layer representing the muscularis propria is intact.
a) Axial plane; b) sagittal planes - lesion length and the distance between the distal tumor edge and the proximal border of the muscles. IAS: internal anal sphincter ; PR: puborectal.
View Figure 2
The results of pathologic and 3D-US analysis are shown for patients with resection without CRT in table 1. Overall agreement was classed as almost perfect (κ = 0.89). For T1, agreement was classed as substantial (κ = 0.77); for T0, T2, and T3, almost perfect (κ = 0.87); and for N1, perfect (κ = 1).
![]()
Table 1: Agreement between pretreatment three-dimentional ultrasound and definitive pathologic tumor categories after resection in 18 patients with rectal cancer without indication for chemoradiation.
View Table 1
Neoadjuvant CRT
Before CRT, 3D-US showed uT2 in 7 patients, uT3 in 63, and uT4 in 8 patients. As noted above, 2 of the patients with uT3 had no clinical indication for CRT and were included in the group who underwent resection only. Thus, 76 patients were assigned to neoadjuvant CRT. Node status was N1 in 38 (50%) patients, of whom 13 had anal canal invasion.
Treatment selection and outcome
Post-CRT examination with 3D-US showed a total of 16 patients with a complete response; of these, 8 were assigned a watch-and-wait strategy (Figure 3 and Figure 4) and 8 underwent sphincter-preserving surgery. The remaining 60 patients had residual tumors and underwent sphincter-preserving surgery or abdominoperineal resection.
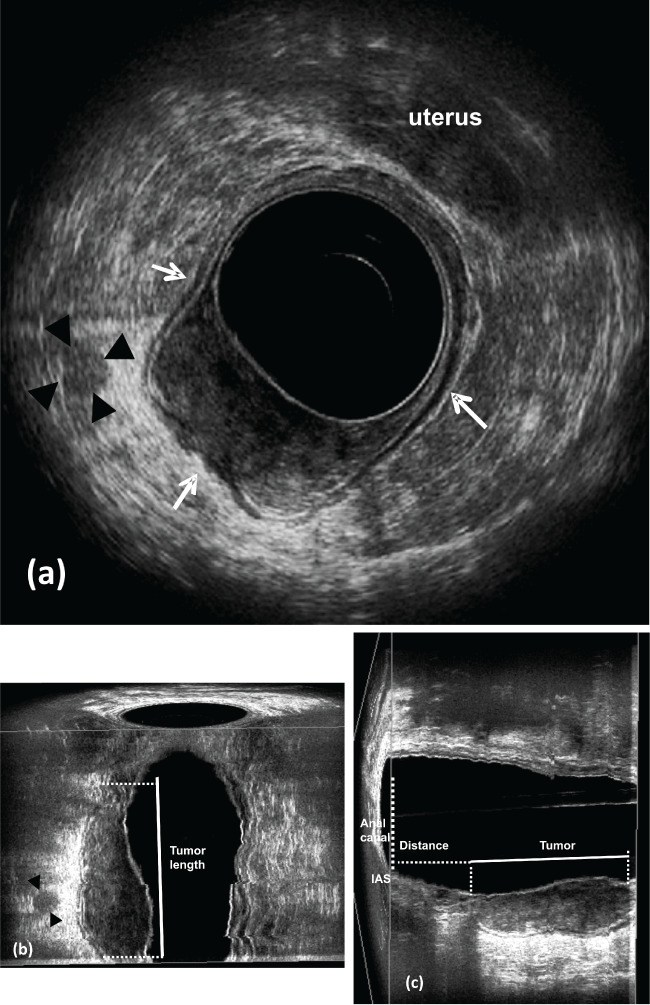
.
Figure 3: Pre CRT.
a) uT3-Rectal cancer located in the right lateral posterior rectal wall. The hyperechoic layer corresponding to the perirectal fat shows irregularity (arrows). Lymph node appears with echogenicity similar to the primary tumor and has a high probability of containing metastatic disease (arrowhead); b) coronal plane - tumor length (arrows); c) sagittal plane - the distance from the distance between the distal tumor edge and the proximal border of the muscles. IAS: internal anal sphincter.
View Figure 3
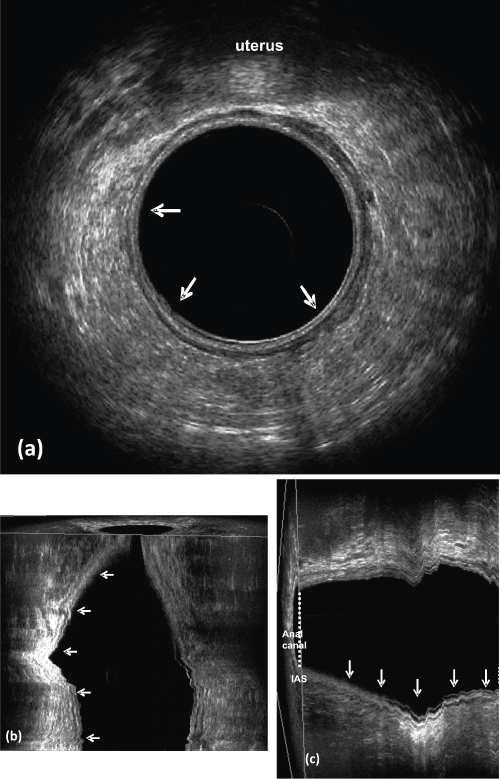
.
Figure 4: Post-CRT (Case Figure 3).
a) Axial plane; b) coronal plane; c) sagittal plane - rectal wall layers are clearly distinguishable at the former tumor location and there is no residual metastatic lymph nodes in the perirectal fat. Pathological examination: the tumor has been eradicated (pT0N0).
View Figure 4
The distance between the distal tumor edge and the proximal border of the muscles was ≥ 1.5 cm in 36 patients (Figure 5 and Figure 6) and < 1.5 cm in 24 patients (Figure 7). The 36 patients with a tumor-muscle distance ≥ 1.5 cm were scheduled for sphincter preservation (including low anterior resection and intersphincteric and/or coloanal anastomosis); however, in 2 of these patients (with a tumor-muscle distance of 1.6 cm), the sphincter could not be saved and they underwent abdominoperineal resection. Conversely, of the 24 patients with a tumor-muscle distance of < 1.5 cm, 22 underwent abdominoperineal resection and 2 patients with a distance of 1 cm underwent the sphincter-preserving procedure. Thus, a total of 44 patients underwent sphincter-preserving surgery (8 with complete response, 34 with a tumor muscle distance ≥ 1.5 cm, and 2 with a distance < 1.5 cm) and a total of 24 patients underwent abdominoperineal resection (22 with a distance < 1.5 cm and 2 with a distance ≥ 1.5 cm).
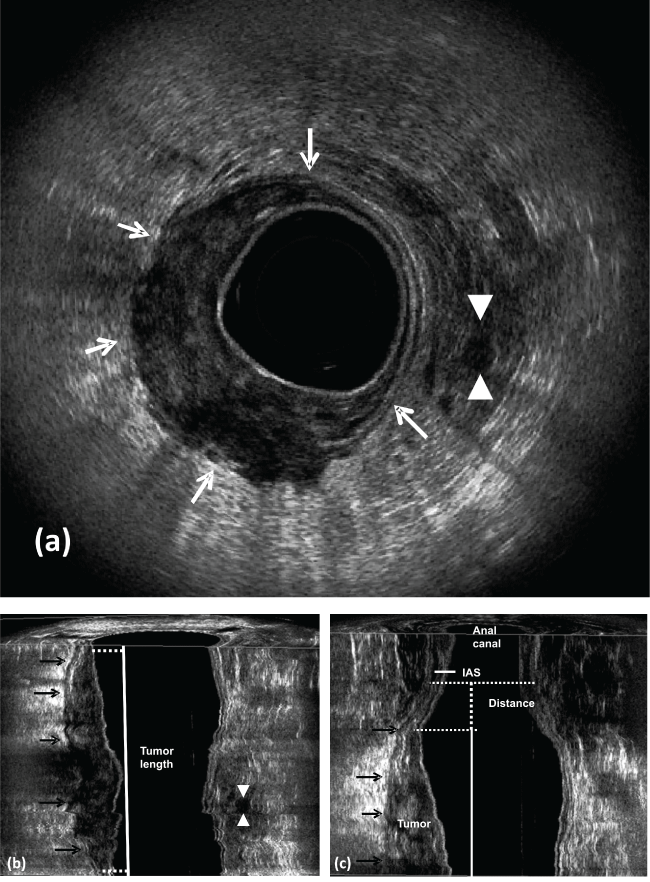
.
Figure 5: Pre CRT.
a) uT3-Rectal cancer occupying half right of the rectal circumference. The hyperechoic layer corresponding to the perirectal fat shows irregularity (arrows). Lymph node appears with echogenicity similar to the primary tumor and has a high probability of containing metastatic disease (arrowhead); b) coronal plane - tumor length (arrows); c) coronal plane - the distance between the distal tumor edge and the proximal border of the muscles. IAS- internal anal sphincter.
View Figure 5
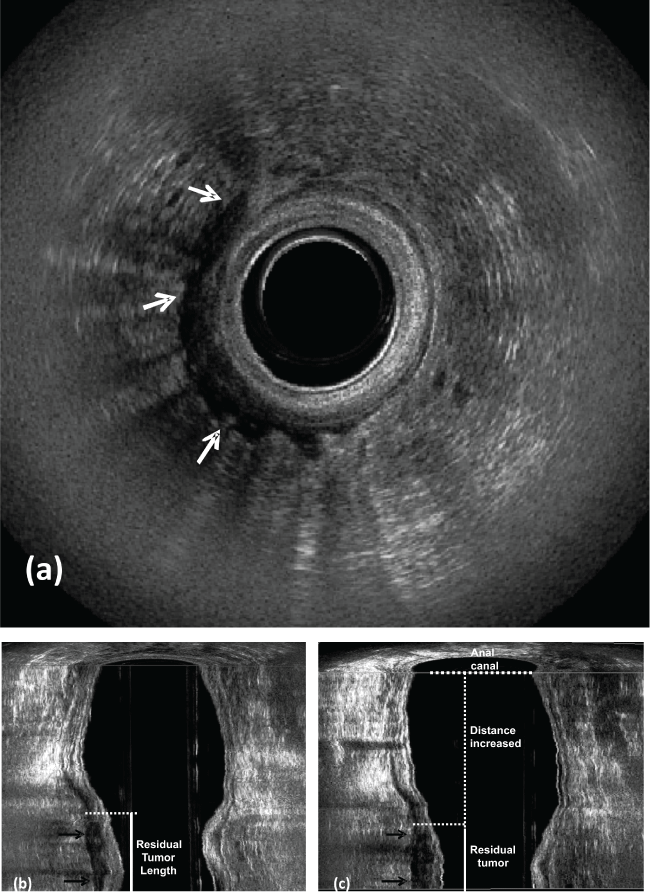
.
Figure 6: Post CRT (Case Figure 5).
a) Residual tumor; b) coronal plane - residual tumor length - reduced (arrows); c) coronal plane - the distance between the distal residual tumor edge and the proximal border of the muscles increased. There is no residual metastatic lymph nodes in the perirectal fat. IAS: internal anal sphincter.
View Figure 6
Of the patients with sphincter-preserving surgery, 3 had recurrence (anastomosis, hepatic, and lung) and 2 were salvaged by surgery. Three patients died, one of hepatic recurrent disease and one of an immediate postoperative complication and another cause. Of the patients with abdominoperineal resection, 3 had recurrence (2 hepatic and 1 pelvic) and 2 had salvage surgery. Two patients died, 1 of pelvic recurrent disease, 1 of another cause. Of the 8 patients with a watch-and-wait strategy, none had recurrence.
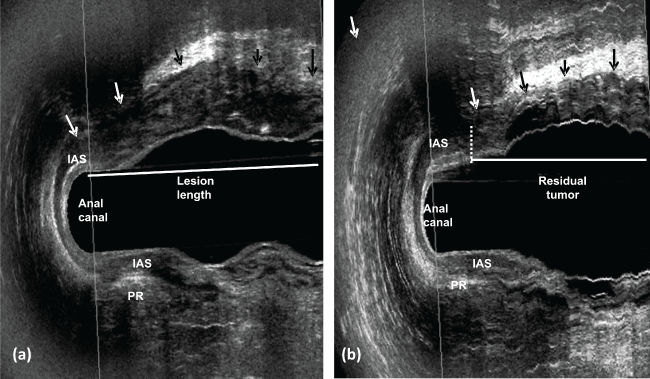
.
Figure 7: a) uT3 - rectal tumor extends from inferior rectum until upper anal canal and invading the internal anal sphincter in the anterior quadrant; b) post CRT - the residual tumor at the same level of the internal anal sphincter in the anterior quadrant (there is no distance between the distal residual tumor edge and the proximal border of the muscles). IAS- internal anal sphincter; PR- puborectal muscle.
View Figure 7
Post-CRT 3D-US showed a significantly shorter tumor length, longer distance between the distal tumor edge and the proximal border, and greater length-reduction ratio in patients selected for sphincter preserving surgery compared with those selected for abdominoperineal resection (Table 2).
![]()
Table 2:Treatment decision and three-dimensional ultrasound measurements in 76 patients 50-55 days after completion of neoadjuvant chemoradiotherapy for rectal cancer.
View Table 2
Agreement: Post-CRT 3D-US vs. pathologic analysis
Results of post-CRT 3D-US were compared with postsurgical pathologic evaluation regarding the identification of residual tumor or complete response (Table 3). The overall agreement was substantial (κ = 0.62). Sensitivity was 98%; specificity, 47%; positive predictive value, 87%; and negative predictive value, 88%. The accuracy of post-CRT 3D-US in identifying residual tumor or complete response was 87% (59/68), with overestimation in 12% (8/68) and underestimation in 1% (1/68). Of the 8 patients with overestimation by post-CRT 3D-US, 7 had sphincter-preserving surgery and 1 had abdominoperineal resection. One patient had underestimation and underwent sphincter-preserving surgery.
![]()
Table 3: Agreement between three-dimentional ultrasound 50-60 days after completion of neoadjuvant chemoradiotherapy and pathologic identification of residual tumor or complete response in the rectal wall.
View Table 3
Results of post-CRT 3D-US were also compared with postsurgical pathologic evaluation regarding lymph node involvement (Table 4). Overall agreement was moderate (κ = 0.42). Sensitivity was 46%; specificity, 92%; positive predictive value, 60%; and negative predictive value, 88%. The accuracy of post-CRT 3D-US in identifying affected lymph nodes was 84% (57/68), with overestimation in 6% (4/68) and underestimation in 10% (7/68). All 4 patients with overestimation by post-CRT 3D-US underwent sphincter preservation; of the 7 patients with underestimation, 5 underwent sphincter preservation and 2 had abdominoperineal resection.
![]()
Table 4: Agreement between post-chemoradiation three-dimensional ultrasound and pathologic findings in the identification of positive and negative lymph nodes.
View Table 4
The pathological examination revealed free distal margins of at least 1.0 cm in all patients.
Agreement: Post-CRT 3D-US vs pathologic analysis
When post-CRT 3D-US results were compared with the combination of pathologic plus clinical response in all patients, including those with the watch-and-wait strategy, agreement was substantial for the identification of residual tumor or complete response (κ = 0.63). Sensitivity was 98%; specificity, 65%; positive, 87% and negative predictive value, 94%. The accuracy of post-CRT 3D-US in identifying residual tumor or complete response was 88% (67/76), with overestimation in 11% (8/76), and underestimation in 1% (1/76).
Regarding lymph nodes, the total agreement between post-CRT 3D-US and pathologic plus clinical response was moderate (κ = 0.42). Sensitivity was 46%; specificity, 94%; positive, 60%; and negative predictive value, 89%. The accuracy of post-CRT 3D-US regarding lymph nodes in all patients was 86% (65/76), with overestimation in 5% (4/76) and underestimation in 9% (7/76).
Discussion
This study demonstrates that the use of the 3D-US to assess rectal cancer can provide preoperative staging, characterize the response after neoadjuvant CRT, and add information that helps in making treatment decisions. For decisions regarding preoperative treatment, for example, neoadjuvant CRT, assessment of the depth of invasion and nodal involvement with 3D-US provides the most important information. However, the ability to measure the length of the tumor and the distance from the tumor to muscles are additional measurements that aid in choosing the best type of resection. Our results showed a high degree of concordance between 3D-US and pathologic analysis for classifying the lesion and identifying involved lymph nodes, similar to previous reports [3-5,20]. Following the protocol in this study, patients were treated with standard CRT and surgical procedures for total mesorectal excision, and the recurrence rate was similar to results in the literature [21].
Various studies have demonstrated reduced accuracy in ultrasound staging after CRT [8-11] restaging the tumor with 3D-US after CRT would not be useful in deciding the best surgical technique and was not used in this protocol. In 2000, Gavioli, et al. [8] used a rigid linear endoprobe to describe features after CRT in patients with rectal tumors and concluded that endorectal ultrasound no longer stages the tumor, but rather the fibrosis that takes its place. These authors showed for the first time that ultrasound can identify lesion regression based on the reappearance of a typical 5-layer stratification at the site of the tumor. Thus, 3D-US is useful after CRT for evaluating criteria such as tumor length, length-reduction ratio, and distance between the distal tumor edge and the proximal border of the sphincter. 3D-US with automatic scanning, high frequency and range of the depth, and multiplane images makes it possible to provide all of these measurements both before treatment and after CRT. This method is well tolerated and is minimally invasive, because the endoprobe is kept stationary during image acquisition.
In previous studies, Murad-Regadas, et al. [12] determined the pattern of ultrasound needed to identify residual lesions and complete response after CRT using 3D-US with automatic scanning. The distance from the tumor to muscles can only be visualized using imaging examinations such as ultrasound [8,12,13]. Measurement from tumor to muscles with digital examination is subjective, and rectosigmoidoscopy measures the distance between the tumor and the pectinate line or anal verge. Even using MRI studies showed limited results; mean sensitivity was 50% and mean specificity 91% to restage the tumor after CRT in systematic review [22]. However, better results were demonstrated when diffusion-weighted imaging and dynamic contrast-enhanced were used [22-24].
In our protocol, the examinations were performed by expert colorectal surgeons with experience in 3D-US and standardized measurements. They agreed on a cutoff point of 1.5 cm for the distance from the tumor to muscles in order to allow sphincter preservation. Most patients with a distance of ≥ 1.5 cm underwent sphincter preservation surgery, and failure to save the sphincter during the surgery occurred in only 2 patients. Furthermore, most patients with < 1.5 cm required abdominoperineal resection, although in 2 patients with a distance of 1 cm it was possible to save the sphincter. The group of colorectal surgeons concluded that 1.5 cm is a sufficient distance, and all patients had this free margin on pathologic analysis.
Half of the patients with complete response underwent sphincter preservation surgery and the other half had a watch-and-wait strategy. Previous studies [8,12,13] have demonstrated good results in determining complete response with ultrasound and have used the results to select patients for this strategy. Only one group of colorectal surgeons in our study adopted the watch-and-wait strategy. However, the sensitivity of post-CRT 3D-US in identifying residual tumor or complete response in the rectal wall was high. Of the 68 patients who underwent surgery, 15 (22%) had a complete response on pathologic analysis: 8 of these patients were overstaged and 7 (10%) were correctly staged by 3D-US. These 7 patients underwent sphincter-preserving surgery. Furthermore, post-CRT 3D-US correctly identified the 8 patients with a complete clinical response who were included in the watch-and-wait strategy.
For lymph node identification, the accuracy of 3D-US was 86%. Sensitivity was low (46%) and specificity was high (94%). Underestimation occurred in 7 patients. This may be because the ability of the endoprobe to evaluate perirectal fat is limited. This method comprises a rigid endocavity probe, and evaluation cannot be performed above the probe position. Studies using diffusion-weighted imaging and dynamic contrast-enhanced MRI in staging lymph node reported accuracy of 84% to 93%, sensitivity of 97%, and specificity of 81% [24-26]. Responses after CRT are heterogeneous and may cause reactive changes such as inflammation and fibrosis. A few cases with excessive inflammatory and fibrotic tissues may cause misinterpretation regarding residual tumor. However, in most cases 3D-US identifies characteristic images of the residual tumor and complete regression of lesions, plus additional measurements.
A limitation of the study was that the assessment of circumferential resection margin using 3D-US was not performed in this protocol. Other studies [27,28] have demonstrated a high grade of concordance when ultrasound was compared with MRI. In particular, for low rectal tumors the accuracy of MRI decreases and that of ultrasound increased [28]. A further study including assessment of circumferential resection margin to evaluate the response after CRT should be conducted to investigate the reproducibility of this method before and after CRT.
Our results demonstrated the reproducibility of the 3D-US standardized measurements across 4 clinics. We expect this initial protocol to be useful in clinical practice to guide the management of rectal tumor patients. In cases with inconclusive results, more than one image may be necessary to decide on an appropriate therapeutic response. It may also be useful to call upon available observers experienced in other methods for evaluating rectal tumor.
In conclusion, rectal cancer was accurately staged and managed using 3D-US. After CRT, 3D-US showed high sensitivity in identifying residual tumor in the rectal wall or complete response and ability to quantify residual tumor. The most important parameter for the selection of patients for sphincter preservation was the distance between the tumor and the sphincter. Safe distal margins should be at least 1.5 cm. 3D-US was a reproducible method for evaluating patients with rectal cancer and enabled following an established protocol with good results.
Source of Funding
No funding was sought or received for this study.
Financial Disclosure
None reported.
References
-
Gualdi GF, Casciani E, Guadalaxara A, d'Orta C, Polettini E, et al. (2000) Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum 43: 338-345.
-
Garcia-Aguilar J, Pollack J, Lee SH, Hernandez de Anda E, Mellgren A, et al. (2002) Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum 45: 10-15.
-
Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, et al. (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 232: 773-783.
-
Giovannini M, Ardizzone S (2006) Anorectal ultrasound for neoplastic and inflammatory lesions. Best Pract Res Clin Gastroenterol 20: 113-135.
-
Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, et al. (2009) How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol 16: 254-265.
-
Zorcolo L, Fantola G, Cabras F, Marongiu L, D'Alia G, et al. (2009) Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc 23: 1384-1389.
-
Li L, Chen S, Wang K, Huang J, Liu L, et al. (2015) Diagnostic Value of Endorectal Ultrasound in Preoperative Assessment of Lymph Node Involvement in Colorectal Cancer: a Meta-analysis. Asian Pac J Cancer Prev 16: 3485-3491.
-
Gavioli M, Bagni A, Piccagli I, Fundaro S, Natalini G (2000) Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer: comparison between sonographic and histopathologic changes. Dis Colon Rectm 43: 1075-1083.
-
Napoleon B, Pujol B, Berger F, Valette PJ, Gerard JP, et al. (1991) Accuracy of endosonography in the staging of rectal cancer treated by radiotherapy. Br J Surg 78: 785-788.
-
Vanagunas A, Lin DE, Stryker SJ (2004) Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol 99: 109-112.
-
Pastor C, Subtil JC, Sola J, Baixauli J, Beorlegui C, et al. (2011) Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum 54: 1141-1146.
-
Murad-Regadas SM, Regadas FS, Rodrigues LV, Barreto RG, Monteiro FC, et al. (2009) Role of three-dimensional anorectal ultrasonography in the assessment of rectal cancer after neoadjuvant radiochemotherapy: preliminary results. Surg Endosc 23: 1286-1291.
-
Murad-Regadas SM, Regadas FS, Rodrigues LV, Crispin FJ, Kenmoti VT, et al. (2011) Criteria for three-dimensional anorectal ultrasound assessment of response to chemoradiotherapy in rectal cancer patients. Colorectal Dis 13: 1344-1350.
-
Hildebrandt U, Feifel G (1985) Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon Rectum 28: 42-46.
-
Regadas FSP, Murad-Regadas SM (2009) Staging and followup of anal canal neoplasms with 2 and 3D ultrasonography. In: Pescatori M, Regadas FSP, Murad-Regadas SM, Zbar AP, Imaging Atlas of the Pelvic Floor and Anorectal Diseases, Springer, Milan, 73-81.
-
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355: 1114-1123.
-
Gerard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, et al. (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24: 4620-4625.
-
Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, et al. (2010) Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum 53: 1692-1698.
-
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37: 360-363.
-
Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G (2009) The Value of High Resolution Three-Dimensional Endorectal Ultrasonography in the Management of Submucosal Invasive Rectal Tumors. Dis Colon Rectum 52: 1837-1843.
-
Topova L, Hellmich G, Puffer E, Schubert C, Christen N, et al. (2011) Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum 54: 401-411.
-
Van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S (2013) Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 269: 101-112.
-
Hötker AM, Garcia-Aguilar J, Gollub MJ (2014) Multiparametric MRI of rectal cancer in the assessment of response to therapy: a systematic review. Dis Colon Rectum 57: 790-799.
-
Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, et al. (2011) Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 18: 2224-2231.
-
Mizukami Y, Ueda S, Mizumoto A, Sasada T, Okumura R, et al. (2011) Diffusion-weighted magnetic resonance imaging for detecting lymph node metastasis of rectal cancer. World J Surg 35: 895-899.
-
Alberda WJ, Dassen HP, Dwarkasing RS, Willemssen FE, van der Pool AE, et al. (2013) Prediction of tumor stage and lymph node involvement with dynamic contrast-enhanced MRI after chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 28: 573-580.
-
Phang PT, Gollub MJ, Loh BD, Nash GM, Temple LK, et al. (2012) Accuracy of endorectal ultrasound for measurement of the closest predicted radial mesorectal margin for rectal cancer. Dis Colon Rectum 55: 59-64.
-
Granero-Castro P, Munoz E, Frasson M, García-Granero A, Esclapez P, et al. (2014) Evaluation of mesorectal fascia in mid and low anterior rectal cancer using endorectal ultrasound is feasible and reliable: a comparison with MRI findings. Dis Colon Rectum 57: 709-714.





