International Journal of Cancer and Clinical Research
Impact of Eribulin Monotherapy on Post-Progression Survival in Patients with HER2-Negative Advanced or Metastatic Breast Cancer
Takeshi Kotake1,2*, Yuichiro Kikawa3, Sachiko Takahara4, Shigeru Tsuyuki5, Hiroshi Yoshibayashi6, Eiji Suzuki2, Yoshio Moriguchi7, Hiroyasu Yamashiro8, Kazuhiko Yamagami9, Hirofumi Suwa10, Toshitaka Okuno11, Takahito Okamura12, Takashi Hashimoto13, Hironori Kato3, Akihito Tsuji1,14 and Masakazu Toi2
1Department of Medical Oncology, Kobe City Medical Center General Hospital, Kobe, Japan
2Breast Surgery Department, Kyoto University Hospital, Kyoto, Japan
3Department of Breast Surgery, Kobe City Medical Center General Hospital, Kobe, Japan
4 Breast Center, Kitano Hospital, Osaka, Japan
5Department of Breast Surgery, Osaka Red Cross Hospital, Osaka, Japan
6Department of Breast Surgery, Japanese Red Cross Wakayama Medical Center, Wakayama, Japan
7Department of Breast Surgery, Kyoto City Hospital, Kyoto, Japan
8Department of Breast Surgery, Tenri Hospital, Nara, Japan
9Department of Breast Surgery, Shinko Hospital, Kobe, Japan
10Department of Breast Surgery, Hyogo Prefectural Tsukaguchi Hospital, Hyogo, Japan
11Department of Breast Surgery, Nishikobe Medical Center, Kobe, Japan
12Department of Breast Surgery, Yamato Takada Municipal Hospital, Nara, Japan
13Hashimoto Clinic, Kobe, Japan
14Department of Clinical Oncology, Faculty of Medicine, Kagawa University, Kagawa, Japan
*Corresponding author:
Takeshi Kotake, Breast Surgery Department, Kyoto University Hospital, 54, Shogoin-Kawaharacho, Sakyo-ku, Kyoto, Japan, Tel: +81-75-751-3660, Fax: +81-75-751-3616, E-mail: tkkotake@gmail.com
Int J Cancer Clin Res, IJCCR-3-061, (Volume 3, Issue 4), Original Article; ISSN: 2378-3419
Received: June 16, 2016 | Accepted: July 22, 2016 | Published: July 25, 2016
Citation: Kotake T, Kikawa Y, Takahara S, Tsuyuki S, Yoshibayashi H, et al. (2016) Impact of Eribulin Monotherapy on Post-Progression Survival in Patients with HER2-Negative Advanced or Metastatic Breast Cancer. Int J Cancer Clin Res 3:061. 10.23937/2378-3419/3/4/1061
Copyright: © 2016 Kotake T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: A multicenter observational retrospective study was conducted to assess the clinical response and survival impact, especially post-progression survival impact, of eribulin monotherapy in HER2-negative advanced or metastatic breast cancer (A/MBC) patients.
Patients and methods: This retrospective observation cohort study contains 110 A/MBC patients treated with eribulin monotherapy during April 2011 and August 2014 in 12 Kyoto Breast Cancer Research Network (KBCRN) institutions. This q3 regime comprised administration of eribulin monotherapy at a starting dose of 1.4 mg/m2 on day 1 and day 8 and repeated every 3 weeks. The all patients were treated on the basis of the attached document of Eribulin. The study endpoints were progression-free survival (PFS), overall survival (OS), and post-progression survival (PPS).
Results: A total of 110 patients were enrolled in the present study. The median PFS and OS were 4.0 months (95% CI, 3.2-4.7) and 16.8 months (95% CI, 8.8-24.7), respectively. The median PPS was significantly longer in patients with increase in size of pre-existing lesion (IPEL group, 12.9 months) than in those with a new lesion or metastasis (NM group, 6.8 months). In multivariate analyses for PPS, the visceral disease, total dose of eribulin and NM were correlated with the PPS (p ≤ 0.001, 0.001 and 0.006, respectively).
Conclusion: The present study indicates the total dose of eribulin and PD type were correlated with the PPS. Suppression of new lesions may lead to the OS-improving effect of eribulin. Further studies are merited to evaluate the effects of this drug on post-treatment.
Keywords
Advanced breast cancer, Chemotherapy, Eribulin, Post-progression survival, Metastasis, Overall survival
Introduction
While survival outcomes of early breast cancer cases have been improved remarkably in recent years, still many patients develop metastases and require the treatment against the relapsed disease. The 10-year survival rate of patients with advanced or metastatic breast cancer (A/MBC) is yet in the low level around 5%, despite the probability of disease silencing differs depending upon tumor subtype [1,2].
Chemotherapy regimens for HER2-negative A/MBC commonly comprise antineoplastic agents such as anthracycline or taxane [3], which provide limited survival advantages. Eribulin is a novel chemotherapeutic agent that inhibits microtubule movement through a site of action different from that of taxanes. Eribulin monotherapy significantly prolonged the overall survival of patients with metastatic breast cancer when compared with the treatment provided by the attending physician, in a phase III study (EMBRACE study) [4]. A randomized trial with capecitabine (301 study) showed a tendency of better overall survival (OS) in patients treated with eribulin.
Based on these findings, eribulin is currently used, for many patients in the clinical practice, as a one of the option in 2nd or 3rd line chemotherapy for HER2-negative A/MBC. It is worth noting that these the aforementioned clinical studies demonstrated prolonged overall survival or a tendency thereof in eribulin-treated patients with no extension in progression-free survival [5]. It was suggested that the treatment with eribulin may reduced occurrence of lethal new metastases such as those in the brain, lung, and liver, and prolong new metastasis-free survival the possible causes [6]. However, the exact underlying mechanisms involved remain largely unknown.
Taking into account from the above, we conducted a multicenter observational retrospective study aiming at assessing the efficacy of eribulin on post-treatment in real-world clinical settings (KBCRN-R01 study).
Patients and Methods
Study population
Cases in which eribulin therapy was introduced for patients with HER2-negative A/MBC, between April 2011 and August 2014, were included in this study. The eribulin-treated cases were retrospectively collected from 12 Kyoto Breast Cancer Research Network (KBCRN) institutions in Japan.
The patients included in the study were histological diagnosed with breast cancer. The tumors were classified as recurrent, locally advanced, metastatic breast cancer using the American Joint Committee on Cancer (7th edition) guidelines. The patients had undergone at least two courses of eribulin therapy, and presented with adequate organ as well as bone marrow functions. Patients were excluded if they had other malignancies or severe complication.
In principle, a 3-week treatment course comprised the administration of eribulin on day 1 and day 8, at a starting dose of 1.4 mg/m2. Depending on patient conditions, the drug was administered at a reduced dose or interrupted at the discretion of the attending physician. Treatment was continued until disease progression, occurrence of a serious toxic reaction, or patient’s request for discontinuation. Attending physicians were allowed to change the method of administration at their discretion when toxic reactions emerged, and administration schedules that allowed for continuation of the treatment were collected. Tumor responses were assessed via CT, their periods depended on the patient’s condition. Data on clinical outcome and toxicity were collected based on retrospective evaluation of the medical records, for the descriptive analyses.
This study was conducted under the approval of the ethics committee from principal institution, taking due care in protecting personal information.
Evaluation of efficacy and safety
Progression free survival (PFS) refers to the period from the start of eribulin administration to progression or death by any cause. Overall survival (OS) is the period from the start of eribulin administration to death, or the last follow-up day. Post-progression survival (PPS) is the period from progression that occurred after eribulin was administered to death, or the last follow-up day. This is the endpoint to evaluate the post-study therapy with eribulin. Overall response rate (ORR) is the proportion of patients for whom complete response (CR) or partial response (PR) was obtained.
The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4) were used for toxicity evaluation, while the conventional Response Evaluation Criteria In Solid Tumors (RECIST) criteria were used to assess the therapeutic effects.
Statistical analysis
Descriptive statistics were computed for demographic, clinicopathologic factors. Pearson χ2-test and Fisher’s exact test were used for comparison of individual variables. Survival data were analyzed using three endpoints (OS, PFS, and PPS), and Kaplan–Meier analysis was performed for each of these subgroups using the log-rank test. Association between risk factors and survival data was examined using the COX proportional-hazards analysis. All statistical analyses were carried out using a computer software (SPSS version, SPSS inc.; Chicago, IL, USA), with p value of ≤ 0.05 considered significant.
Results
Patient characteristics
A total of 110 patients with A/MBC underwent eribulin therapy at 12 participating institutions in Japan between April 2011 and August 2014. Table 1 illustrates the background of the patients included in the study. The patients with A/MBC received a median of 3 chemotherapy lines, wherein 86 (78.2%) and 104 (94.5%) of them had been previously treated with anthracycline-and taxane-based anti-cancer agents, respectively.
![]()
Table 1: Patient characteristics.
View Table 1
Eribulin was discontinued in 99 (90.0%) patients due to increase in size of pre-existing lesion (IPEL) in 57 (51.8%) patients, appearance of a new lesion or metastasis (NM) in 31 patients (28.2%), toxicity in 8 patients (7.3%), and other reasons in 3 (2.7%) patients. The other reasons included progression of a co-existing disease (2 patients) and elevated tumor markers (1 patient).
Efficacy results
The median observation period was 16.7 months (range, 8.8-24.7 months). At the time of final observation, 11 (10.0%) patients were receiving eribulin, and 63 (57.3%) patients were alive. Of the 110 patients enrolled, CR and PR were obtained in 1 (0.9%) and 20 (18.2%) patients, respectively, with an ORR of 19.0%.
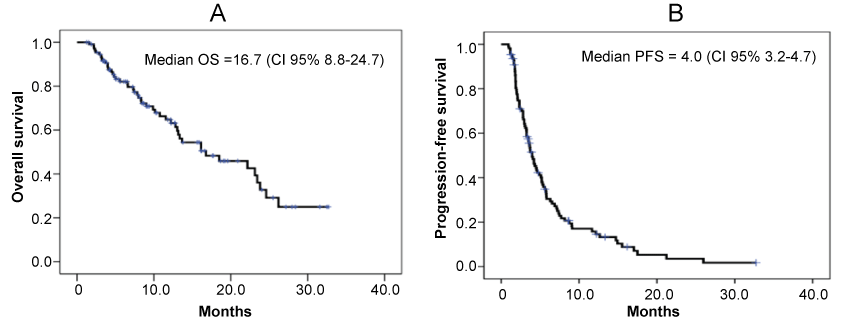
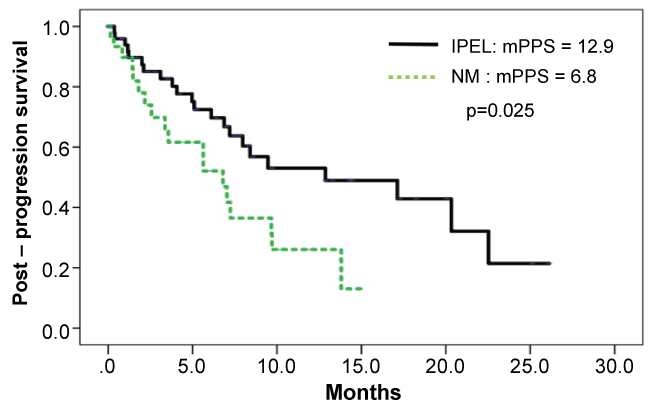
The median PFS was 4.0 months (95% CI, 3.2-4.7), while median OS was 16.8 months (95% CI, 8.8-24.7) (Figure 1). PPS was analyzed in 88 patients with tumor progression following eribulin treatment. Table 2 illustrates the background of the patients with tumor progression. From Kaplan–Meier analysis, the PPS for 57 patients with IPEL and for 31 patients with NM were 12.9 months (95% CI, 2.1-23.6) and 6.8 months (95% CI, 3.2-10.3), respectively (p = 0.025) (Figure 2).
.
Figure 2: A Kaplan-Meier curves of post-progression survival (PPS) stratified by PD in 88 patients who had visceral disease when eribulin therapy was started. Increase in the size of pre-existing lesion (IPEL) vs. Appearance of a new lesion or metastasis (NM).
View Figure 2
Moreover, multivariate analyses for PPS were conducted for age, subtype, the presence or absence of distant organ metastasis, line of eribulin administration, total dose of eribulin and PD type. The visceral disease, total dose ≥ 10 mg/m2 and NM were found to correlate with the PPS (p ≤ 0.001, 0.001 and 0.006, respectively) (Table 3).
![]()
Table 2: Characterisics of patients with tumor progression following eribulin treatment.
View Table 2
![]()
Table 3: Univariate and multivariable cox regression analyses of PPS.
View Table 3
Discussion
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) guidelines generally recommend the continuous administration of a single drug chemotherapy for HER2-negative A/MBC patients [7,8]. However, although anthracycline- and taxane-based anti-cancer agents have been shown to be efficacious [9,10], still it is unclear about the magnitude of therapeutic impact on OS [11-13]. Eribulin monotherapy is one of the few chemotherapy regimens that have been proven to prolong the OS. In the EMBRACE study, median PFS and OS were 3.7 months and 13.2 months, respectively [4]. In the present study, the median PFS and OS were 4.0 months (95% CI, 3.2-4.7) and 16.8 months (95% CI, 8.8-24.7), respectively. Thus, major therapeutic outcomes of the this study reproduced or even compared favorably with the results from the EMBRACE study.
The essential aims of the treatment for A/MBC patients are to improve survival outcomes and maintain quality of life (QOL). To this end, it is important to manage drug toxicity, which, as a result, contributes not only to improvement in QOL but also to treatment continuity. In this study, 8 out of 110 cases (7.3%) where eribulin was discontinued due to the toxicity. In a retrospective study conducted by Dranitsaris et al., the rate of toxicity-caused discontinuation with eribulin was 8.9%, which was lower than that reported for other drugs [14]. These findings indicate that eribulin can be safely and effectively administered to breast cancer patients.
The two previous studies (EMBRACE and 301) showed a unique property of eribulin treatment where PFS was not improved by the treatment but OS was significantly. The OS prolongation effect of eribulin was also reported in the treatment of progressive soft-tissue sarcomas. A phase III study, showed a similar trend that OS was significantly better in the eribulin-treated group compared with the standard treatment group, while no significant difference of PFS was detected between these two groups [15].
Perez et al. have reported that, in the 301 study that compared capecitabine with eribulin, lethal organ metastases, such as those in the liver, the lung and the central nervous system, occurred at a lower frequency and after a longer period of time in the eribulin group [6]. Further, Yoshida et al. have shown in an experimental setting that eribulin suppresses metastasis of breast cancer cells by inducing the conversion of epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) (appendix equation) [16]. EMT is an essential cell function for tissue construction during normal developmental [17] and is also observed during cancer progression; it is considered to promote invasion and metastasis by enhancing the motility of cancer cells and degradation of extracellular matrix [18].
In the present study, total dose of eribulin and appearance of a new lesion or metastasis (HR, 0.26; p = 0.001, HR, 2.84; p = 0.006, respectively) were factors that showed statistically significant correlations with PPS in the multivariate analysis. This suggests that eribulin may suppress new lesions and lead to improved prognosis. However, such a prognosis-improving effect of eribulin should be compared with other anti-cancer agents, and is just a speculation at this point of time. Further investigations on the OS-improving effect of eribulin through prospective studies and translational research are expected.
Conclusion
The present study indicates the total dose of eribulin and appearance of a new lesion were correlated with the PPS. Suppression of new lesions may lead to the OS-improving effect of eribulin. Further studies are merited to evaluate the effects of this drug on post-treatment.
Ethical Statement
The study was done in accordance with the Declaration of Helsinki and the regulations of the Japanese Ministry of Health, Labour and Welfare. The study protocol was approved by the ethics committee of Kobe City Medical Center General Hospital.
References
-
Greenberg PA, Hortobagyi GN, Smith TL, Ziegler LD, Frye DK, et al. (1996) Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 14: 2197-205.
-
Rahman ZU, Frye DK, Smith TL, Asmar L, Theriault RL, et al. (1999) Results and long term follow-up for 1581 patients with metastatic breast carcinoma treated with standard dose doxorubicin-containing chemotherapy: a reference. Cancer 85: 104-111.
-
Katsumata N, Watanabe T, Minami H, Aogi K, Tabei T, et al. (2009) Phase III trial of doxorubicin plus cyclophosphamide (AC), docetaxel, and alternating AC and docetaxel as front-line chemotherapy for metastatic breast cancer: Japan Clinical Oncology Group trial (JCOG9802). Ann Oncol 20: 1210-1215.
-
Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, et al. (2011) Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377: 914-923.
-
Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, et al. (2012) A phase III, open-label, randomized, multicenter study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes. CTRC-AACR San Antonio Breast Cancer Symposium.
-
EA Perez (2013) New metastasis versus increase in size of pre-existing lesion and its correlation with overall survival in patients with MBC treated with eribulin of capecitabine in Study 301, a Phase III randomized trial. ECC, 1911.
-
Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, et al. (2014) Chemotherapy and Targeted Therapy for Women With Human Epidermal Growth Factor Receptor 2-Negative (or unknown) Advanced Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 32: 3307-3329.
-
Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, et al. (2014) Breast cancer version 3.2014. J Natl Compr Canc Netw 12: 542-590.
-
Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, et al. (1998) Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. J Clin Oncol 16: 3439-3460.
-
Ghersi D, Wilcken N, Simes RJ (2005) A systematic review of taxane-containing regimens for metastatic breast cancer. Br J Cancer 93: 293-301.
-
Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kölbl H, et al. (2003) Multicenter phase II study of oral capecitabine (Xeloda(")) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol 14: 1227-1233.
-
Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, et al. (1995) Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol 13: 2731-2736.
-
Toi M, Saeki T, Aogi K, Sano M, Hatake K, et al. (2005) Late phase II clinical study of vinorelbine monotherapy in advanced or recurrent breast cancer previously treated with anthracyclines and taxanes. Jpn J Clin Oncol 35: 310-315.
-
Dranitsaris G, Beegle N, Kalberer T, Blau S, Cox D, et al. (2014) A comparison of toxicity and health care resource use between eribulin, capecitabine, gemcitabine, and vinorelbine in patients with metastatic breast cancer treated in a community oncology setting. J Oncol Pharm Pract 21: 170-177.
-
Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, et al. (2016) Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 387: 1629-1637.
-
Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, et al. (2014) Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer 110: 1497-1505.
-
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119: 1438-1449.
-
Floor S, van Staveren WC, Larsimont D, Dumont JE, Maenhaut C (2011) Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. Oncogene 30: 4609-4621.