International Journal of Cancer and Clinical Research
Improved Antitumoral Efficacy of Mesothelin Targeted Immune Activating Fusion Protein in Murine Model of Ovarian Cancer
Yang Zeng1, Binghao Li1, Patrick Reeves1, Chongzhao Ran2, Zhao Liu1, Mojgan Agha-Abbaslou1, Jianping Yuan1, Pierre Leblanc1, Ann Sluder1, Jeffrey Gelfand1, Timothy Brauns1, Mark Poznansky1 and Huabiao Chen1*
1Vaccine and Immunotherapy Center, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA
2Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA
*Corresponding author:
Huabiao Chen, Vaccine and Immunotherapy Center, Massachusetts General Hospital (East), 149 13th Street, Charlestown, MA 02129, USA, Tel: 617-643-2561, Fax: 617-726-5411, E-mail: Huabiao.chen@mgh.harvard.edu
Int J Cancer Clin Res, IJCCR-3-051, (Volume 3, Issue 2), Research Article; ISSN: 2378-3419
Received: February 15, 2016 | Accepted: March 28, 2016 | Published: March 30, 2016
Citation: Zeng Y, Li B, Reeves P, Ran C, Liu Z, et al. (2016) Improved Antitumoral Efficacy of Mesothelin Targeted Immune Activating Fusion Protein in Murine Model of Ovarian Cancer. Int J Cancer Clin Res 3:051. 10.23937/2378-3419/3/2/1051
Copyright: © 2016 Zeng Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The candidate therapeutic fusion protein (scFv-MtbHsp70) is a recombinant Mycobacteria tuberculosis heat shock protein 70 (MtbHsp70) fused with a single chain antibody (scFv) targeting mesothelin, combining the immune-targeting capacity of the scFv with the broader immune activating capabilities of MtbHsp70. The previous version of the fusion protein, VIC-007, markedly enhanced survival of ovarian tumor-bearing mice through the augmentation of tumor-specific cell-mediated immune responses. In this study, a new version of the fusion protein, VIC-008, was reconstructed from VIC-007 by slight modifications that removed redundant amino acids, and introduced a single amino acid mutation, phenylalanine to valine, at position 381 of MtbHsp70 to prevent non-specific peptide binding and presentation while retaining immune-stimulatory capacity. VIC-008 showed significantly improved protection in control of tumor growth and prolongation of the survival of tumor-bearing mice compared to VIC-007. These findings offered a definitive preclinical validation of VIC-008 as a therapeutic agent for ovarian cancer.
Keywords
Mycobacterial Hsp70, Mesothelin, Single Chain Variable Fragment, Ovarian Cancer
Abbreviations
MSLN: mesothelin; scFv: single-chain antibody variable fragment; MTB: Mycobacterium tuberculosis; Hsp: heat shock protein; DC: dendritic cell; i.p.: intraperitoneal.
Introduction
Ovarian cancer is the most lethal malignancy in women, with 22,280 new cases and 15,460 deaths estimated in the United States in 2012 [1]. Surgical debulking followed by platinum-based chemotherapy remains the current standard-of-care treatment to which approximately 80% of patients respond favorably. However, more than 60% of patients who initially achieve remission eventually relapse and less than 40% survive beyond 5 years [2]. Thus, novel treatments for ovarian cancer are urgently needed. Although clinical trials of various immunotherapeutic modalities have not yet yielded significant benefit [3], the failures to date can be understood in light of the complexities of immune regulation, potentially leading to the development of more efficacious immunotherapies.
A novel fusion protein, VIC-007, consists of the broadly immune-activating Mycobacterium tuberculosis-derived heat shock protein 70 (MtbHsp70) and the tumor antigen targeting activity of a single-chain variable fragment (scFv) binding mesothelin (MSLN), a validated immunotherapy target [4-6]. MSLN is highly over expressed on the surface of common epithelial cancers including epithelial malignant mesothelioma and ovarian cancer, while expressed at relatively low levels only in mesothelial cells lining the pleura, pericardium, and peritoneum in healthy individuals [7-10]. MtbHsp70 is well characterized and functions as a potent immune-activating adjuvant. It stimulates monocytes and dendritic cells (DCs) to produce CC-chemokines [11,12], which attract antigen processing and presenting macrophages, DCs, and effector T and B cells [13]. In theory, fusion of anti-MSLN scFv and MtbHsp70 takes advantage of the immune-activating action of MtbHsp70 and the tumor-targeting activity of the scFv, which will yield anti-tumor responses against the broadest profile of tumor antigens.
Although our previous studies showed that VIC-007 significantly enhanced survival of immune competent mice with ovarian or malignant mesothelioma tumors through the augmentation of tumor-specific cell-mediated immune responses [14], the fusion protein did not result in long-term remission. In this study a new version of the fusion protein, VIC-008, was reconstructed from VIC-007 to remove redundant amino acids and minimize the activity of the natural peptide-binding site of MtbHsp70. We compared VIC-007 and VIC-008 side by side in the same set of mice and found that VIC-008 conferred significantly improved antitumoral efficacy in a syngeneic, orthotopic and immune competent murine model of ovarian cancer.
Materials and Methods
Cells
The ID8 ovarian cancer cells, a kind gift from Kathy Roby (University of Kansas Medical Center, Kansas City, KS) [15], were transfected with luciferase lentiviral vector and stably expressed luciferase, here named Luc-ID8. Cells were maintained at 37°C in DMEM with 2 mmol/L L-glutamine, 10 units/ml penicillin, 10 μg/ml streptomycin, and 10% fetal bovine serum in humidified atmosphere with 5% CO2. Cells were cultured until 80% confluent, and harvested with Trypsin EDTA (Mediatech) for animal injections.
Animal model and treatment
Ovarian cancer was established by Intraperitoneal (i.p.) injection of syngeneic cancer cells Luc-ID8 (5 × 106 cells per mouse) into 6-week-old female C57BL/6 mice. All mice were purchased from Jackson laboratories. Mice with ovarian tumors were treated 7 days after tumor cell inoculation with i.p. injections of VIC-007 (4 μg per mouse), VIC-008 (4 μg per mouse), or normal saline. This was followed by 3 further treatments at 7-day intervals. All studies were performed in a manner that was blinded to the observer under protocols that were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (SRAC).
In vivo imaging of tumor growth
Intraperitoneal tumor growth was monitored weekly after tumor cell inoculation using in vivo live imaging by IVIS Spectrum (PerkinElmer). Mice were injected intraperitoneally with 150 mg/kg body weight of D-luciferin 10 min in advance and subsequently imaged by IVIS Spectrum.
Mouse survival
For survival studies, we observed the mice daily 1 week after inoculation of tumor cells. Tumor generations were consistently first evident via abdominal distension secondary to malignant ascites, and tumor-bearing mice were euthanized at the endpoint when there were signs of distress, including fur ruffling, rapid respiratory rate, hunched posture, reduced activity, and progressive ascites formation as previously described [16].
Statistical analysis
Statistical differences between three or more experimental groups were analyzed using Two-Way ANOVA, followed by Tukey's multiple comparison tests when mean of each group is compared with that of every other group. Survival was analyzed with the Log-rank test. Prism 6.0 software (GraphPad Software) was used for all the statistical analysis.
Results and Discussion
Reconstruction of the fusion protein scFv-MtbHsp70
The fusion protein scFv-MtbHsp70 was constructed with VH and VL from anti-MSLN p4 scFv [17] fused to full length MtbHsp70 with a (G4S)3 linker in between, which has been shown in our previous study [14]. The previous version of the fusion protein VIC-007 achieved significant control of tumor growth and prolongation of the survival of tumor-bearing mice, but the antitumoral efficacy of the treatment regimen used needed to be improved. Antigenic peptides linked to MtbHsp70 through both non-covalent binding and by genetic fusion can elicit both MHC class I-restricted CD8+ and MHC class II-restricted CD4+ T-cell responses [18-22]. In this study we developed a new version of the scFv-MtbHsp70 fusion protein, VIC-008, which was modified from the original VIC-007 by the elimination of redundant amino acids and the introduction of a single amino acid mutation, phenylalanine (F) in place of valine (V), at position 381 of MtbHsp70 (Figure 1). This change is designed to prevent peptide binding [23] while retaining the immune-stimulatory capacity of the protein, in order to reduce the possibility that MtbHsp70 might incidentally bind and deliver other antigens that could result in off target effects or the induction of tolerance or antoimmunity.

.
Figure 1: Protein reconstruction. Amino acid sequences of VIC-007 and VIC-008 were shown. VIC-008 was reconstructed from VIC-007 by removal of redundant amino acids GSS, SGILEQQG, and AAAMRS indicated in bold and italic and introduction of a single amino acid mutation, phenylalanine (F) in place of valine (V), at position 381 of MtbHsp70.
View Figure 1
The fusion proteins were constructed and expressed by WuXi App Tech (Shanghai, China) in CHO cells and provided at a purity of above 95% by HPLC and an endotoxin level of less than 1.0 EU/mg.
VIC-008 enhances the control of tumor growth
Murine ovarian cancer was established by i.p. injection of syngeneic cancer cells Luc-ID8 in immune competent C57BL/6 mice and treated with VIC-007 and VIC-008 as described in the section of materials and methods. As shown in figure 2, both VIC-007 and VIC-008 significantly slowed tumor growth as recorded by bioluminescence signals compared to saline (p < 0.0001 and p < 0.0001) while VIC-008 further significantly delayed tumor growth compared to VIC-007 (p < 0.0001).
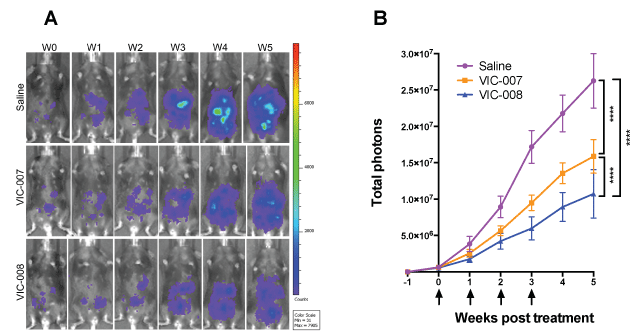
.
Figure 2: Tumor growth. Quantitative analysis of bioluminescence signals was performed using IVIS Spectrum on Luc-ID8 tumor inoculated mice (n = 8 or 9) at a week after tumor inoculation and subsequently weekly. (A) Longitudinal images of a representative mouse from each treatment group were presented from a week after tumor inoculation before treatment (W0) and subsequent five weeks (W1 – W5); (B) The arrows indicated 4 treatments at 7-day intervals starting at a week after tumor inoculation. Total photons were calculated by IVIS Lumina Series III. Statistical differences were analyzed using Two-Way ANOVA followed by Tukey's multiple comparison tests. ****, p < 0.0001. Data were indicated as the mean ± sem.
View Figure 2
VIC-008 enhances the prolongation of mouse survival
We further evaluated the efficacy of VIC-007 and VIC-008 to prolong survival in the tumor-bearing mice. As shown in figure 3, both VIC-007 and VIC-008 significantly enhanced the survival of tumor-bearing mice compared to saline (p = 0.0253 and p = 0.0002) with increased median survival of 55 days from saline to 60 days from VIC-007 and further to 65 days from VIC-008. VIC-008 further significantly prolonged the survival of the tumor-bearing mice compared to VIC-007 (p = 0.0301).
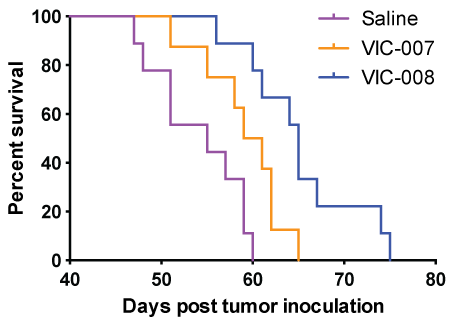
.
Figure 3: Mouse survival. The mice were observed daily 1 week after treatment. At the endpoint the mice were euthanized and the survival time were calculated as life span from the day of tumor inoculation. The median survival and p values were determined using the Log-rank test.
View Figure 3
Taken together, these data showed that the new version of the fusion protein VIC-008 significantly delayed the tumor growth and prolonged the survival in a syngeneic murine model of ovarian cancer. Improved mouse survival of VIC-008 compared to VIC-007 is likely related to the changes made to the protein sequences. Since the efficacy of immunotherapeutic agents is often limited by negative immunological regulations that attenuate immune responses in the tumor microenvironment when used as a single agent, we will conduct further testing of VIC-008 in combination with checkpoint blockage that are capable of reprogramming suppressive and stimulatory signals in the intratumoral environment that together with VIC-008 has the potential to yield more powerful cancer control.
Conclusion
Our study provides a definitive preclinical validation of the mesothelin targeted immune activating fusion protein as a therapeutic agent for ovarian cancer and supports the continued exploration of this novel fusion protein alone or in combination with immune checkpoint inhibitors or chemotherapy for MSLN-expressing cancers.
Acknowledgments
This work was supported by the VIC Mesothelioma Research and Resources Program and the Friends of VIC Fund, and the Edmund C. Lynch, Jr. Cancer Fund.
Competing Interests
We declare that we have no competing financial interests.
References
-
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, et al. (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62: 220-241.
-
Bast RC, Hennessy B, Mills GB (2009) The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9: 415-428.
-
Mantia-Smaldone GM, Corr B, Chu CS (2012) Immunotherapy in ovarian cancer. Hum Vaccin Immunother 8: 1179-1191.
-
Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, et al. (2010) Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 16: 6132-6138.
-
Hassan R, Ho M (2008) Mesothelin targeted cancer immunotherapy. Eur J Cancer 44: 46-53.
-
Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I (2009) Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res 15 :5274-5279.
-
Chang K, Pastan I (1996) Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A 93: 136-140.
-
Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, et al. (2001) Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 7: 3862-3868.
-
Ho M, Bera TK, Willingham MC, Onda M, Hassan R, et al. (2007) Mesothelin expression in human lung cancer. Clin Cancer Res 13: 1571-1575.
-
Tang Z, Qian M, Ho M (2013) The role of mesothelin in tumor progression and targeted therapy. Anticancer Agents Med Chem 13: 276-280.
-
Floto RA, MacAry PA, Boname JM, Mien TS, Kampmann B, et al. (2006) Dendritic cell stimulation by mycobacterial Hsp70 is mediated through CCR5. Science 314: 454-458.
-
Wang Y, Kelly CG, Karttunen JT, Whittall T, Lehner PJ, et al. (2001) CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15: 971-983.
-
Baggiolini M (1998) Chemokines and leukocyte traffic. Nature 392: 565-568.
-
Yuan J, Kashiwagi S, Reeves P, Nezivar J, Yang Y, et al. (2014) A novel mycobacterial Hsp70-containing fusion protein targeting mesothelin augments antitumor immunity and prolongs survival in murine models of ovarian cancer and mesothelioma. J Hematol Oncol 7: 15.
-
Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, et al. (2000) Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 21: 585-591.
-
Righi E, Kashiwagi S, Yuan J, Santosuosso M, Leblanc P, et al. (2011) CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res 71: 5522-5534.
-
Bergan L, Gross JA, Nevin B, Urban N, Scholler N (2007) Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett 255: 263-274.
-
Udono H, Srivastava PK (1993) Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med 178: 1391-1396.
-
Suto R, Srivastava PK (1995) A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science 269: 1585-1588.
-
Suzue K, Young RA (1996) Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol 156: 873-879.
-
Huang Q, Richmond JF, Suzue K, Eisen HN, Young RA (2000) In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4(+) T cell independent. J Exp Med 191: 403-408.
-
Ciupitu A-MT, Petersson M, O'Donnell CL, Williams K, Jindal S, et al. (1998) Immunization with a Lymphocytic Choriomeningitis Virus Peptide Mixed with Heat Shock Protein 70 Results in Protective Antiviral Immunity and Specific Cytotoxic T Lymphocytes. J Exp Med 187: 685-691.
-
MacAry PA, Javid B, Floto RA, Smith KG, Oehlmann W, et al. (2004) HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity 20: 95-106.





