International Journal of Cancer and Clinical Research
HER2 Positive Gastric And Gastroesophageal Adenocarcinoma; An Irish Tertiary Center Experience
Yasir Y Elamin1*, Shereen Rafee1, Mutaz M Nur2, Nemer Osman1, John V Reynolds3, Cian Muldoon2 and Kenneth J O'Byrne1
1Department of Medical Oncology, St James's Hospital, Ireland
2Department of Pathology, St James's Hospital, Ireland
3Department of General Surgery, St James's Hospital, Ireland
*Corresponding author: Yasir Y Elamin, Medical Oncology Department, St James's Hospital, Dublin 8, Ireland, Tel +353 872 659 665, E-mail: Elaminy@tcd.ie
Int J Cancer Clin Res, IJCCR-1-008, (Volume 1, Issue 1), Research Article; ISSN: 2378-3419
Received: September 10, 2014 | Accepted: October 27, 2014 | Published: October 29, 2014
Citation: Elamin YY, Rafee S, Nur MM, Osman N, Reynolds JV, et al. (2014) HER2 Positive Gastric And Gastroesophageal Adenocarcinoma; An Irish Tertiary Center Experience. Int J Cancer Clin Res 1:008. 10.23937/2378-3419/1/1/1008
Copyright: © 2014 Elamin YY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Trastuzumab has been approved for patients with human epidermal growth factor receptor 2 (HER2) over expression and gene amplification metastatic gastric cancer. Here we present the prevalence of HER2 positive gastric cancer in an Irish population, the use of Trastuzumab in first line and beyond progression.
Methods: The study was conducted in St James's Hospital, Dublin. A retrospective analysis of the date of patients with HER2 positive gastric cancer over a period of 3 years was carried out. Her2 positive was defined as immunohistochemistry (IHC) score of +3, of IHC score of +2 and increased gene copy number by fluorescence in situ hybridization (FISH). Overall survival was calculated from the day of initiation of treatment with Trastuzumab until death.
Results: During the study period 140 patients with gastric and gastro-esophageal junction adenocarcinoma were treated. Out of those, 30 (21.4%) had HER2 positive disease. Among HER2 positive disease patients 18 (12.8%) were treated with first line Trastuzumab containing regimen with a median overall survival of 13 months. Nine (50%) developed progressive disease while on Trastuzumab and of those, 4 (22.2%) patients continued on Trastuzumab beyond progression, two (11.1%) of whom achieved stable disease and a prolonged survival.
Conclusion: HER2 positivity rate in an Irish population with advanced gastric and gastro-esophageal junction adenocarcinoma is 21.4%. Treatment with Trastuzumab in the first line in combination with chemotherapy is a reasonable approach. Continuation of Trastuzumab beyond progression is a feasible strategy that requires further exploration.
Keywords
Targeted therapy, Metastatic gastric cancer, Her2, Trastuzuma
Introduction
Human epidermal growth factor receptor-2 (HER2) is a member of the epidermal growth factor receptor family1. HER2 is a transmembrane tyrosine kinase receptor coded by the ERBB gene on chromosome 17. It plays an important role in regulating cell survival and growth by acting through the PI3K/PTEN/AKT and RAS-MAPK pathways [1]. HER2 overexpression and gene amplification are assessed by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) respectively, on biopsies or surgical specimens, and have been described in a number of human tumors including breast, gastric, and endometrial cancers [2]. Therefore, it is an appealing target in the treatment of these malignancies.
HER2 overexpression/gene amplification is observed in up to 30% of breast cancers [3]. Positivity is associated with aggressive disease and a worse prognosis when compared to HER2 negative breast cancer. Trastuzumab is a monoclonal antibody that targets HER2, and has been shown to prolong overall survival and progression free survival in early and metastatic breast cancer in a number of clinical trials [1, 3].
The role of HER2 in gastric and gastro-esophageal junction adenocarcinoma is less well defined, with reported overexpression rates varying between 8 to 50 % of cases [2]. Equally, data on its prognostic value is not consistent. The ToGA trial, a phase III randomized clinical trial reported in 2010, investigated the role of Trastuzumab in patients with HER2 positive metastatic gastric and gastro-esophageal junction adenocarcinoma [4]. The addition of Trastuzumab led to an increase in the median overall survival by 2.7 months compared to standard chemotherapy alone.
Our understanding of the biological role of HER2 overexpression/gene amplification in gastric cancer and the therapeutic implications is still evolving. We report the results of screening for HER2 overexpression/gene amplification in patients with advanced gastric and gastro-esophageal cancers in a tertiary institute; we further discuss their treatment and survival.
Methods
The study was conducted at St James University Hospital, a tertiary referral center for esophageal and gastric cancer. Patients included in the study had metastatic or inoperable gastric or gastro-esophageal junction adenocarcinoma and treated in the period between April 2010 and April 2013.
Retrospective review of prospectively collected patients data was carried out, data including patients demographics, disease staging, histological subtype, treatment and survival were gathered from patients electronic and written records.
HER2 positivity was defined as IHC +3 or IHC +2 and FISH positive [2,4,5]. HER2 negativity was defined as IHC 0/1 (1+), or IHC +2 and FISH negative. Median overall survival was calculated for patients treated with Trastuzumab from the day of treatment initiation with Trastuzumab until death. Data was censored on the first of May 2013. Data on compliance with treatment, treatment toxicity, and treatment interruptions were available for all patients treated in our center. While on Trastuzumab, Patients cardiac function was monitored by echocardiogram or Multi Gated Acquisition Scan (MUGA) every three months. Treatment toxicity was graded according to the National Cancer Institute common toxicity criteria version 4.0. Statistical analysis was carried out using GraphPad Prism 6®.
Results
During the study period, 162 patients presented with metastatic or inoperable gastric and gastro-esophageal junction adenocarcinoma, of these 140 (86.4%) were assessed for HER2 protein overexpression/gene amplification. Sixty one (43.6%) had gastric adenocarcinoma and 79 (56.4%) had gastro-esophageal junction adenocarcinoma. All patients samples were assessed using immunohistochemistry (HerceptTest, Dako, Denmark or PATHWAY anti-HER-2 (4B5), Ventana, Tucson, AZ. USA). Only samples with IHC score of +2 were assessed subsequently with FISH, results of which are shown in Table 1.
![]()
Table 1: Results of HER2 assessment.
View Table 1
Thirty (21.4%) patients were found to have HER2 positive gastric or gastro-esophageal adenocarcinoma; their characteristics are outlined in Table 2. Of these, 18 were treated with a Trastuzumab containing regimen. 12 patients did not receive Trastuzumab as part of their treatment. The reasons for this were: 6 elected to be treated in another institute, 5 were deemed unfit for systemic therapy, and 1 patient declined treatment. Among the 18 patients who did receive Trastuzumab, the drug was given in combination with Oxaliplatin and a fluoropyrimidine (either Capecitabine or Fluorouracil) in 9. HER2 status was unavailable at the time of treatment initiation in 6 patients, whose initial treatment therefore consisted of Epiduracin, Oxaliplatin and a fluoropyrimidine (either Capecitabine or Fluorouracil). Following one or two cycles, when HER2 status was subsequently reported, Epiduracin was replaced with Trastuzumab. The remaining 3 received Trastuzumab in combination with flurouracil.
![]()
Table 2: HER2 positive patient characteristics.
View Table 2
In the cohort treated with Trastuzumab, 9 patients developed progressive disease while on treatment, and 5 of them were deemed unfit and died without receiving additional therapy. 4 continued on Trastuzumab beyond progression and among these, the accompanying regimen was switched from an initial combination of Oxaliplatin and a fluoropyrimidine to Docetaxel in three, and one received radiotherapy in combination.
The median overall survival for patients treated with a Trastuzumab containing regimen was 13 months (range 1-36 months) (Figure 1). The mean number of Trastuzumab cycles given was 11.5 (range 1-49). No grade 3 or 4 cardiac toxicities occurred in patients treated with Trastuzumab.
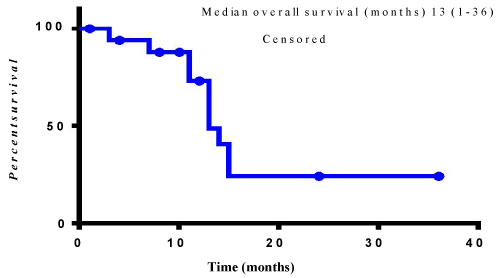
Figure 1: Median overall survival of HER2 positive patients treated with Trastuzumab.
View Figure 1
Discussion
Gastric cancer is the second most common cause of cancer related deaths worldwide [6]. Combination chemotherapy remains an integral part of the treatment of both locally advanced and metastatic gastric cancer with modest improvement in survival rates when compared to other common malignancies such as breast and colorectal cancer [7]. Therefore, there is still a pressing need to develop novel therapeutic agents. Targeting HER2 in a selected cohort of patients promises to be an effective strategy.
Of our study population, 21.4% were HER2 positive, comparable to 22.1% reported in patients screened for eligibility in the ToGA trial [4]. It is important to note that both our cohort and that of the ToGA trial included patients with metastatic disease. Recent publications where only patients with early or locally advanced gastric and gastro-esophageal junction carcinoma were included, showed rates of (Her-2 Positivity of) 10.4% in the MAGIC trail cohort and 10.9% in the INT-0116 trial [8,9]. A potential source of bias in our study is that data was collected from a single institute. However, our center is the largest gastric cancer center in Ireland; we have screened 86.4% of all patients with metastatic gastric cancer. Therefore, it is likely that the rate of 21.4% that we found in this study cohort is a reflection of HER2 positivity rate in Irish patients with this disease.
In accordance with European medicines agency (EMEA) definition for Trastuzumab approval, we defined HER2 positive disease as IHC +3 or IHC+2 and FISH positive in both biopsies and surgical specimens. This definition varies from that of the FDA, which further differentiates between biopsies and surgical specimens. The FDA defines a HER2 positive biopsy as one that is IHC+3, or IHC+2 and FISH positive. A HER2 positive surgical specimen, however, is one that is either IHC+3, or FISH positive regardless of IHC score [8]. Most studies reported a concordance rate between IHC and FISH of 90-98% [8-11].
The authors of the ToGA trial, the only large phase III trial thus far to report on HER2 positive gastric cancer, took a more liberal stance with regard to entry criteria, with 20% of their patients being FISH positive with IHC scores of 0 and 1 [4]. As previously mentioned, the ToGA trial reported a survival benefit of 2.7 months with a regimen combining cisplatin, a fluoropyrimidine and Trastuzumab compared to a regimen of cisplatin and a fluoropyrimidine alone. However, a preplanned analysis of patients with the above definition of HER2 positive disease (IHC+3 or IHC+2 and FISH positive) showed a survival benefit of 4.2 months. The results of the ToGA trial are the basis of Trastuzumab approval in HER positive gastric and gastro-esophageal adenocarcinoma. Our practice has consistently been to treat patients with HER2 positive inoperable or metastatic gastric and gastro-esophageal adenocarcinoma with a combination of Trastuzumab, oxaliplatin, and a fluoropyrimidne. In some cases, where the HER2 status is yet to be reported, we initiate treatment with oxaliplatin, a fluoropyrimidine, and epiduracin. We replace the latter with Trastuzumab in cases of HER2 positivity. Although epiduracin has been combined safely with Trastuzumab in advanced breast cancer, there is no data to support such combination in gastric cancer [7,11].
A unique feature of our study was the continuation of Trastuzumab beyond progression. Trastuzumab was continued with second line therapy in four patients. Two of them achieved stable disease with prolonged survival of 3 years in one patient and 1.1 years in the other. The best evidence for the benefit of continuing Trastuzumab beyond progression comes from studies done on breast cancer, where clinical data show significant benefit in both Phase III studies and retrospective series [12-15]. To date there are no studies that have evaluated this approach in gastric cancer, however, a number of prospective clinical trials are currently underway to clarify this matter [2].
Several publications examined the prognostic and predictive role of HER2 overexpression/gene amplification in gastric cancer and have reported inconsistent results [2,7,16,17]. Recently, Prins et al. reported that HER2 positivity predicted poor survival in a cohort of 144 patients with locally advanced esophageal adenocarcinoma [11]. However, the authors used tissue microarrays (TMA) obtained from surgical specimens in their assessment of HER2 status. TMA generation carries a risk of sampling error with reported false negative rates of up to 24% [18]. In contrast, retrospective review of the MAGIC trial cohort showed that HER2 positivity did not predict the survival of 415 patients with locally advanced gastric or gastro-esophageal adenocarcinoma [8]. In addition, the researchers concluded that HER2 positivity did not predict response to epiduracin as was the case in breast cancer [8].
Several questions related to HER2 in gastric and gastro-esophageal cancer remain unanswered. What is the optimal definition of HER2 positive disease in biopsies and surgical specimens? Does HER2 status change following chemotherapy? What is the role of Trastuzumab in the adjuvant setting and in the metastatic setting when there is progression while on treatment? These questions are currently being addressed in a number of phase III clinical trials [2,8,11].
Novel HER2 targeting agents include Lapatinib, an oral tyrosine kinase inhibitor that blocks HER2 downstream signaling [19]. The efficacy of Lapatinib in HER2 positive gastro-esophageal cancer is currently being tested in a phase III clinical trial [20]. Also, Pertuzumab, a monoclonal antibody that prevents HER2 dimerization, is in the final stages of clinical studies, where it is combined with Trastuzumab and standard chemotherapy [21].
Other targeted therapies include monoclonal antibodies that target the epidermal growth factor receptor (EGFR), namely Cetuximab and Panitumumab, which were investigated in gastric and esophageal cancer with disappointing results [22,23]. Bevacizumab, an anti-vascular endothelial growth factor receptor (VEGF) monoclonal antibody, increased progression free survival when added to standard chemotherapy in metastatic gastric cancer, as shown in the AVAGAST trial [24]. However, the AVAGAST study did not meet its primary endpoint which was overall survival. The role of Bevacizumab in early and localized gastro-esophageal cancer is being examined in an ongoing phase III clinical trial [25].
In summary, we have shown that the HER2 positivity rate in an Irish population with advanced gastric and gastro-esophageal junction adenocarcinoma is 21.4%. Treatment with Trastuzumab achieved a median survival of 13 months. Continuation of Trastuzumab beyond progression is a feasible strategy that requires further exploration.
References
-
Hudis CA (2007) Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 357: 39-51.
-
Boku N (2013) HER2-positive gastric cancer. Gastric Cancer 17: 1-12.
-
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, et al. (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14: 320-368.
-
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 376: 687-697.
-
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, et al. (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52: 797-805.
-
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24: 2137-2150.
-
Okines AF, Cunningham D (2010) Trastuzumab in gastric cancer. Eur J Cancer 46: 1949-1959.
-
Okines AF, Thompson LC, Cunningham D, Wotherspoon A, Reis-Filho JS, et al. (2013) Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol 24: 1253-1261.
-
Gordon MA, Gundacker HM, Benedetti J, Macdonald JS, Baranda JC, et al. (2013) Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann Oncol 24: 1754-1761.
-
Prins MJ, Ruurda JP, van Diest PJ, van Hillegersberg R, Ten Kate FJ (2013) The significance of the HER-2 status in esophageal adenocarcinoma for survival: an immunohistochemical and an in situ hybridization study. Ann Oncol 24: 1290-1297.
-
Okines A, Cunningham D, Chau I (2011) Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 8: 492-503.
-
Mohd Sharial MS, Crown J, Hennessy BT (2012) Overcoming resistance and restoring sensitivity to HER2-targeted therapies in breast cancer. Ann Oncol 23: 3007-3016.
-
von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, et al. (2011) Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer 47: 2273-2281.
-
Mannocci A, De Feo E, de Waure C, Specchia ML, Gualano MR, et al. (2010) Use of trastuzumab in HER2-positive metastatic breast cancer beyond disease progression: a systematic review of published studies. Tumori 96: 385-391.
-
Murphy CG, Fornier M (2010) HER2-positive breast cancer: beyond trastuzumab. Oncology (Williston Park) 24: 410-415.
-
Aizawa M, Nagatsuma AK, Kitada K, Kuwata T, Fujii S, et al. (2014) Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer 17: 34-42.
-
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP (2011) Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology 59: 832-840.
-
Lordick F, Röcken C (2013) The identification of predictive factors for perioperative chemotherapy in esophago-gastric cancer. Ann Oncol 24: 1135-1138.
-
Khasraw M, Bell R (2012) Primary systemic therapy in HER2-amplified breast cancer: a clinical review. Expert Rev Anticancer Ther 12: 1005-1013.
-
GlaxoSmithKline (2014) LOGiC - Lapatinib Optimization Study in ErbB2 (HER2) Positive Gastric Cancer: A Phase III Global, Blinded Study Designed to Evaluate Clinical Endpoints and Safety of Chemotherapy Plus Lapatinib.
-
Hoffmann-La Roche (2013) A Study of Perjeta (Pertuzumab) in Combination With Herceptin (Trastuzumab) and Chemotherapy in Patients With HER2-Positive Metastatic Gastroesophageal Junction or Gastric Cancer.
-
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, et al. (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14: 490-499.
-
Okines AF, Ashley SE, Cunningham D, Oates J, Turner A, et al. (2010) Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol 28: 3945-3950.
-
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, et al. (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29: 3968-3976.
-
Okines AF, Langley RE, Thompson LC, Stenning SP, Stevenson L, et al. (2013) Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: a safety report. Ann Oncol 24: 702-709.





