The objective of this study is to investigate pre-intervention IVUS characteristics of in-stent restenosis (ISR) lesion correlated with PEB failure.
We performed pre-intervention IVUS for 58 patients with ISR and treated with PEB angioplasty. The PEB failure was defined as death, myocardial infarction and symptom driven revascularization. IVUS images were analyzed at 6 locations: Proximal and distal vessel segment within 3 mm from stent edge, proximal and distal stent edge, lesion site at minimal lumen area and minimal stent area.
Among 58 patients treated with 58 PEB (3.0 ± 0.4 mm by 21.9 ± 4.8 mm), PEB failure were developed at 8 patients (13.8%) during 0.81 years of follow-up. ISR of drug eluting stent comprised large proportion (86.2%) among index procedure. There was no significant difference in clinical presentation and used stents at index procedure between PEB failure and non-failure group. In PEB failure group, neointimal area (4.0 ± 0.7 vs. 2.9 ± 0.8 mm2, p = 0.05) and neointimal hyperplasia (%) (59.4 ± 12.0 vs. 46.0 ± 24.9%, p = 0.05) were significant greater than non-failure group. Stent under-expansion was found in 24 ISR lesions (48.0%) of non-failure group, whereas none was detected in PEB failure group.
PEB failure for ISR lesion could be associated with prominent neointimal hyperplasia on normally extended stent. Future studies are warranted to investigate another treatment modality that might substitute PEB in the treatment of ISR lesions with those characteristics.
Intravascular ultrasound (IVUS), Coronary restenosis, Neointimal hyperplasia, Drug-eluting balloon (DEB), Paclitaxel
In-stent restenosis (ISR) remains unsolved issue in the field of interventional cardiology, though drug-eluting stents (DES) has markedly reduced the occurrence. There is no gold standard in the treatment of ISR, drug-eluting balloons (DEB) has emerged as alternative interventional device to DES.
Paclitaxel-eluting balloons (PEB) angioplasty has been reported to be superior to plain old balloon angioplasty (POBA) and non-inferior to paclitaxel-eluting stents (PES) implantation for treatment of bare metal stent (BMS) restenosis [1-6]. PEB angioplasty has been shown to be superior to POBA for treatment of DES restenosis [7,8]. However, an incidence of target lesion revascularization rate has been reported as 10% at 12 or 24 months after PEB angioplasty (range 4 to 20%) [9-11]. Although PEB has shown notable clinical outcomes in recent trials, no sufficient information exists on the underlying mechanisms of treatment with PEB. Intravascular ultrasound (IVUS) can be used to identify mechanisms of ISR, for example, intimal hyperplasia, under-expansion of stent and geographic miss such as edge problem [12].
To our knowledge, there is no systemized report for IVUS characteristics of PEB failure in the treatment of ISR. The objectives of this study were to investigate pre-intervention IVUS characteristics of ISR lesion correlated with PEB failure and further to suggest as predictors of clinical outcomes.
In this multicenter prospective observational study, we evaluated patients with newly detected ISR on follow-up coronary angiography among patients who previously have received coronary stent. The diagnosis of ISR was determined by > 50% of percent diameter stenosis at coronary angiogram. We excluded patients with left main disease and graft vessel disease and lesions that were pre-dilated before IVUS, lesions treated with stent implantation by operator's decision and follow up duration less than 6 months.
Patients were categorized into 2 groups according to the presence or absence of event after PEB angioplasty treatment: Non-PEB failure group vs. PEB failure group.
This study was notified and approved by the institutional ethics committee, and all patients provided written informed consent.
Baseline clinical data were obtained by an experienced research nurse at the time of the procedure. Demographic and clinical information included gender, age, presence of hypercholesterolemia (under treatment or < 240 mg/dl), diabetes mellitus (dietary glycemic control or oral hypoglycemic agent or insulin-treated), hypertension. Data on previous coronary intervention included clinical presentation (angina or myocardial infarction), stent type, stent diameter and length. All patients were interviewed at clinics and contacted by phone on clinical status minimum 6 months after the procedure.
Patients who had ISR lesion on conventional coronary angiography underwent PEB (SeQuent Please®, B Braun, Melsungen, Germany) angioplasty. The technique for the ISR lesion treatment was a stepwise strategy according to the "stenting" approach. Before PEB angioplasty, we performed pre-PCI IVUS and subsequent plaque or stent modification with POBA. The modification was done with 0.5 mm smaller diameter of conventional balloon than that of the PEB. And then, PEB angioplasty was performed with 30 seconds of inflation time as recommended. Special care was taken to avoid balloon inflation on normal segment and potential geographical miss. The procedure was completed when the result met the criteria of angiographic success (TIMI 3 flow in the main and with a diameter stenosis < 10% respectively).
All patients were treated with aspirin 100 mg and a 300 mg loading-dose of clopidogrel within 12 hours before procedure, respectively. Heparin was administered intravenously to maintain an activated clotting time > 250 sec during the procedure. Administration of glycoprotein IIb/IIIa inhibitors was left to the physician's discretion. Aspirin was continued indefinitely after the procedure and clopidogrel (75 mg/day) was maintained to months only.
All IVUS studies were performed after intracoronary administration of 200 µg of nitroglycerin using a commercial IVUS scanner (Boston Scientific Corporation, Maple Grove, MN and or Volcano Corporation, CA, USA). The IVUS catheter was advanced beyond the target ISR lesion followed by automatic transducer pull back (at 0.5 mm/second) to the proximal reference vessel of ISR lesion. IVUS images were recorded digitally onto a CD or DVD for offline analysis.
IVUS images were analyzed at 6 locations: Proximal and distal reference segment within 3 mm beyond the stent edge, proximal and distal segment of stent, the site of the narrowest neo-intimal lumen (minimal lumen area, MLA), and the site of the narrowest stent area (minimal stent area, MSA), respectively. Proximal and distal reference segments were the most normal looking cross sections within 3 mm beyond the stent edge, and proximal and distal segment of stent were most normal looking cross sections of proximal and distal half of stent, respectively.
Qualitative analysis was performed blind to clinical data according to the criteria of the American College of Cardiology clinical expert consensus document on IVUS. Using the planimetry software (TapeMeasure®, INDEC Systems, Mountain View, California), we measured external elastic membrane (EEM) CSA (cross sectional area)(mm2), lumen CSA (mm2) and stent area (SA, mm2). Plaque burden, defined as plaque and media (P&M) CSA, was calculated as EEM CSA minus lumen CSA [EEM CSA- lumen CSA, (mm2)]. Percent plaque burden was calculated as P&M CSA divided by EEM CSA [(EEM CSA-lumen CSA)/ EEM CSA x 100, (%)].
Neo-intimal CSA was calculated as stent area (SA) minus neo-intimal lumen CSA [SA-neo-intimal lumen CSA(mm2)]. Percentage of neointimal hyperplasia was calculated as neo-intimal CSA divided by SA [(SA-neo-intimal lumen CSA)/SA × 100, (%)]. Stent under-expansion was defined if SA was less than 5 mm2.
PEB failure was the primary outcome of this study, defined if any major adverse cardiovascular events (MACE) occurred during follow-up period. A composite of MACE was defined as all deaths, any myocardial infarction, symptom driven target vessel revascularization including percutaneous coronary intervention and coronary artery bypass surgery.
Continuous variables were presented as the mean ± standard deviations and compared with student unpaired t test. Categorical variables were expressed as frequencies and percentages and compared with chi-square test. P values < 0.05 were considered significant. Statistical analysis was performed with Stat-View 5.0 (SAS Institute, Cary, North Carolina).
Between October 2011 and October 2013 a total of 58 consecutive patients treated with PEB [diameter: 3.0 ± 0.4, length: 21.9 ± 4.8 mm)] for ISR were included in this study. The mean age of population was 64.5 ± 11. 4 years, and 46 (79.3%) were male.
Clinical presentations at index procedure were various: 30 patients presented as stable angina, 20 patients as unstable angina and 8 patients as non-ST-segment elevation myocardial infarction (NSTEMI). There were 8 patients (13.8%) with BMS restenosis, 26 patients (44.8%) with first generation DES restenosis and 24 patients (41.4%) with second generation DES restenosis, with the mean duration from previous PCI to PEB angioplasty, as 9.4 years, 3.1 years and 2.65 years, respectively.
PEB failure was developed at 8 patients (13.8%) after PCI, and the mean duration from PCI to PEB angioplasty was 0.81 years (range, 0.5 years to 2.1 years). The baseline clinical demographics, procedural characteristics of index procedure and PEB angioplasty, and clinical outcomes of each group are presented in Table 1.
Table 1: Distribution of baseline clinical characteristics, procedural characteristics of index procedure and paclitaxel-eluting balloon (PEB) angioplasty, and clinical outcomes according to the presence or absence of events after PEB angioplasty (n = 58). View Table 1
There were no differences in the sex (40 (80%) vs. 6 (75%); p = 0.62), age (64.7 ± 12.1 vs. 63.5 ± 7.2; p = 0.10), cardiovascular risk factors; diabetes (p = 0.74), hypertension (p = 0.16), dyslipidemia (p = 0.48), prior angina or myocardial infarction (p = 0.24) and chronic kidney disease (p = 0.86) between PEB failure and non-failure group. Variables on index procedures were similar between two groups, including clinical presentation (p = 0.24), index stent type (p = 0.68), diameter (p = 0.87) and length (p = 0.43). Parameters on PEB procedure did not show statistical differences on diameter (3.0 ± 0.4 vs. 3.1 ± 0.3; p = 0.53), length (22.0 ± 5.0 vs. 21.5 ± 3.0; p = 0.43) and duration from index PCI to PEB angioplasty (0.74 vs. 1.0, years; p = 0.06) (Table 1).
Grayscale IVUS quantitative measurements at the proximal reference segment, proximal segment of stent, distal segment of stent and distal reference segment were assessed consecutively in order; EEM CSA of each region was 13.9, 13.6, 12.4 and 11.8 mm2, respectively, lumen CSA assessed in each region were 6.31, 5.12, 5.34 and 6.0 mm2, respectively. EEM CSA and lumen CSA of each region were not statistically different between two groups. Pre-procedural IVUS findings of each groups are shown in Table 2.
Table 2: Intravascular Ultrasound (IVUS) findings according to the presence or absence of events after PEB angioplasty (n = 58). View Table 2
In lesions with stent; proximal segment of stent, minimum lumen site, minimum stent site and distal segment of stent, neointimal CSA was 1.41, 3.74, 3.05 and 1.14 mm2, respectively. Minimum lumen site and minimum stent site had much more plaque burden then stent edges. Neointimal plaque burden (presented as neointimal CSA in Table 2) of minimum lumen site and minimum stent site were greater in PEB failure group than in non-PEB failure group, though it failed to demonstrate statistically significant difference.
In PEB group, neointimal hyperplasia was greater than in non-PEB group, especially that of minimum stent site showed statistical difference (59.4 ± 12.0 vs. 46.0 ± 24.9%, p = 0.04) , though that of minimum lumen site failed to show statistical difference (p = 0.48).
Meanwhile, stent under-expansion was more common in non-PEB failure group than in PEB failure group (24 (48%) vs. 0 (0%); p = 0.02).
Figure 1 shows a representative case of ISR lesion with stent under-expansion with modest plaque burden which was successfully treated with PEB. Representative cases of PEB failure with normal expansion of stent and large plaque are presented in Figure 2 and Figure 3.
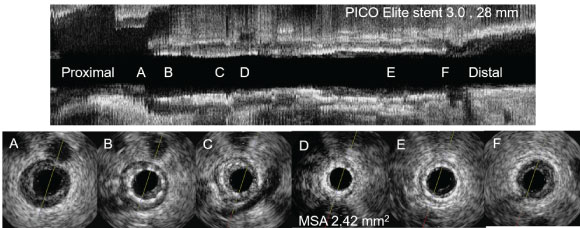 Figure 1: IVUS findings of ISR lesion with stent under-expansion. B,C,D) Pre-PCI IVUS for ISR lesion showed stent under expansion with modest plaque burden; This lesion was treated with Paclitaxel-eluting balloons (3.0 × 26 mm) angioplasty, the patient was uneventful during 1.9 years of follow-up period. ISR: In-stent restenosis; PCI: Percutaneous coronary intervention; IVUS: Intravascular ultrasound; MSA: Minimal stent area.
View Figure 1
Figure 1: IVUS findings of ISR lesion with stent under-expansion. B,C,D) Pre-PCI IVUS for ISR lesion showed stent under expansion with modest plaque burden; This lesion was treated with Paclitaxel-eluting balloons (3.0 × 26 mm) angioplasty, the patient was uneventful during 1.9 years of follow-up period. ISR: In-stent restenosis; PCI: Percutaneous coronary intervention; IVUS: Intravascular ultrasound; MSA: Minimal stent area.
View Figure 1
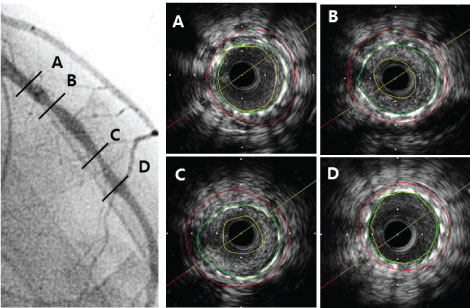 Figure 2: Representative case of ISR lesion of LAD with normally expanded stent and large plaque burden. B,C) Pre-PCI IVUS for ISR lesion showed typical normal expansion and large plaque burden; This lesion was treated with Paclitaxel-eluting balloons (3.0 × 26 mm) angioplasty, he died of sudden death 1.12 years after PEB. ISR: In-stent restenosis; LAD: Left anterior descending artery; PCI: Percutaneous coronary intervention; IVUS: Intravascular ultrasound.
View Figure 2
Figure 2: Representative case of ISR lesion of LAD with normally expanded stent and large plaque burden. B,C) Pre-PCI IVUS for ISR lesion showed typical normal expansion and large plaque burden; This lesion was treated with Paclitaxel-eluting balloons (3.0 × 26 mm) angioplasty, he died of sudden death 1.12 years after PEB. ISR: In-stent restenosis; LAD: Left anterior descending artery; PCI: Percutaneous coronary intervention; IVUS: Intravascular ultrasound.
View Figure 2
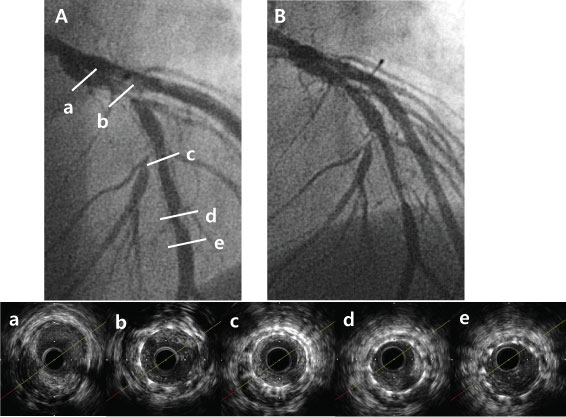 Figure 3: Representative case of ISR lesion with normally expanded stent and large plaque burden. A) Pre-PCI CAG revealed diffuse ISR of left anterior descending artery; b,c,d). Pre-PCI IVUS for ISR lesion showed typical normal expansion and large plaque burden; This lesion was treated with PEB (3.0 × 20 mm) angioplasty Significant restenosis was noticed at PEB site on follow up CAG (B) 0.9 year after PEB due to ischemic chest pain. Target lesion revascularization with coronary artery bypass surgery was performed. ISR: In-stent restenosis; PCI: Percutaneous coronary intervention; CAG: Coronary angiography; IVUS: Intravascular ultrasound; PEB: Paclitaxel-eluting balloon.
View Figure 3
Figure 3: Representative case of ISR lesion with normally expanded stent and large plaque burden. A) Pre-PCI CAG revealed diffuse ISR of left anterior descending artery; b,c,d). Pre-PCI IVUS for ISR lesion showed typical normal expansion and large plaque burden; This lesion was treated with PEB (3.0 × 20 mm) angioplasty Significant restenosis was noticed at PEB site on follow up CAG (B) 0.9 year after PEB due to ischemic chest pain. Target lesion revascularization with coronary artery bypass surgery was performed. ISR: In-stent restenosis; PCI: Percutaneous coronary intervention; CAG: Coronary angiography; IVUS: Intravascular ultrasound; PEB: Paclitaxel-eluting balloon.
View Figure 3
The present study investigated the IVUS characteristics predicting PEB failure in the treatment of ISR. In this study, burden of neointimal hyperplasia was higher and under-expansion of index stent was not observed in PEB failure group. On the contrary in non-PEB failure group, neointimal hyperplasia was even modest and stent under-expansion was more commonly present. This suggests that the pathophysiological mechanisms of PEB failure for ISR lesion could be associated with prominent neo-intimal hyperplasia (and its evolution) on normally extended stent.
The mechanisms of lumen gain obtained with drug-eluting balloons (DEB) are similar to those obtained by POBA, except properties regarding paclitaxel. In a previous IVUS study on POBA, Mehran R, et al. has reported that the mechanisms of balloon angioplasty were additional stent expansion and tissue extrusion out of the stent [13]. And in recent study by Alfonso, et al., mechanisms of lumen gain after balloon angiography were demonstrated as decreased neointimal volume and stent expansion [14]. In the current study, stent under-expansion was detected frequently in non-PEB failure group, whereas none was detected in PEB failure group. This finding corresponded well to the findings of above studies. It is intuitive that PEB angioplasty can be successful in ISR lesions with stent under-expansion, because correction of under-expansion can be achieved easily by balloon angioplasty.
Regarding the neointimal hyperplasia, recent study has shown significant decrease in neointimal volume 6 month after PEB angioplasty for BMS ISR [15]. This effect might be caused by immediate direct mechanical effect of balloon angioplasty as mentioned above, and by the drug effect continuously after the intervention. Paclitaxel has been reported to have properties of rapid uptake and prolonged retention, and to cause apoptosis and necrosis of endothelial and smooth muscle cell [16]. In order that, drug delivery to the smooth muscle cell is important to achieve proper pharmacological effect on smooth muscle and to avoid endothelial toxicity.
On the contrary, recent studies have shown delayed neointimal formation after PEB therapy in DES ISR lesions [7,17]. This finding is similar to previous studies on DES, in which delayed neointimal proliferation had been reported, including experimental animal studies [18-20]. One of the possible explanations of this phenomenon is persistent drug effect-related inflammatory response caused by the drug/polymer combination [18,21]. In the current study, significant pre-procedural neointimal hyperplasia was demonstrated in PEB-failure group. Taking into account that the ISR of DES comprised a large proportion of the study population, this could be interpreted as that, delayed neointimal formation followed by DES has been progressed after PEB.
Future studies are warranted to investigate another PCI modality that might substitute PEB in the treatment of ISR lesions with prominent neointimal hyperplasia on normally extended stent.
In our study, post-procedural IVUS and follow-up IVUS were not performed to all patients. Changes of luminal, stent, neointimal CSA among pre-intervention, post-intervention and follow-up would provide information on nature of neointimal hyperplasia after PEB angioplasty.
Additionally in this study, only quantitative analysis on grayscale IVUS of neointimal atherosclerosis was performed. Plaque composition analysis on neointimal atherosclerosis was not performed. Recently published optical coherence tomography study on DES ISR, ISR lesions with homogeneous neointima were associated with greater subsequent regrowth of neointima compared to lesions with non-homogeneous neointima [17]. Similar approach on IVUS findings of neointimal atherosclerosis would benefit to predict clinical outcome after PEB angioplasty.
Lastly, relatively small number of patients had clinical event in current study. The low number of events resulted in limited study power to find statistically significant clinical correlates of PEB failure was difficult. Therefore it requires a multicenter trial to produce more robust results.
This report demonstrated the IVUS characteristics predicting PEB failure in the treatment of ISR. PEB angioplasty can be successful in ISR lesions with stent under-expansion, and can be unsuccessful in ISR lesions with significant neointimal hyperplasia on normally extended stent. Another PCI modality is required that might benefit in the treatment of ISR lesions with prominent neointimal hyperplasia on normally extended stent.
None.
This study was supported by a grant from the Korean Society of Interventional Cardiology [# 2010-2013].