International Journal of Clinical Cardiology
Relationship between Insulin Levels and Coronary Atherosclerosis in Newly Diagnosed Diabetes Mellitus and Impaired Glucose Toleranc
Naohiko Nemoto*, Rintarou Nakajima, Kennji Ymazaki, Makoto Utsunomiya, Masaki Hori, Shingo Ito, Itaru Yokouchi, Masamichi Wada, Masanori Shiba, Hisao Hara, Hidehiko Hara, Takuro Takagi, Kaoru Sugi and Masato Nakamura
Division of Cardiovascular Medicine, Ohashi Hospital, Toho University Medical Center, Japan
*Corresponding author: Naohiko Nemoto, Division of Cardiovascular Medicine, Ohashi Hospital, Toho University Medical Center, 2-17-6 Ohashi Meguro-ku 153-8515, Tokyo, Japan, Tel: +81 33468 1251, Fax +81 3 3468 1269, E-mail: naohiko-0620@nifty.com
Int J Clin Cardiol, IJCC-2-020, (Volume 2, Issue 1), Review Article; ISSN: 2378-2951
Received: December 25, 2014 | Accepted: February 03, 2015 | Published: February 06, 2015
Citation: Nemoto N, Nakajima R, Ymazaki K, Utsunomiya M, Hori M, et al. (2015) Relationship between Insulin Levels and Coronary Atherosclerosis in Newly Diagnosed Diabetes Mellitus and Impaired Glucose Tolerance. Int J Clin Cardiol 1:020. 10.23937/2378-2951/1410020
Copyright: © 2015 Nemoto N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: While some therapies implemented for newly diagnosed Diabetes Mellitus (DM) or impaired Glucose Tolerance (IGT) are effective in preventing cardiovascular events, investigations of coronary plaque in patients with newly diagnosed DM or IGT are lacking.
Methods: We evaluated 96 patients with acute coronary syndrome. The External Elastic Membrane (EEM), Lumen Cross-Sectional Area (CSA), plaque CSA, and plaque burden in the Left Anterior Descending (LAD) artery proximal to the lesion, diagnosed as normal by Coronary Angiography (CAG), were measured using Intravascular Ultrasound (IVUS). Patients were divided according to DM status (DM=35, non-DM=61). Non-DM patients underwent a 75g Oral Glucose Tolerance Test (OGTT) and were further divided into abnormal glucose tolerance (AGT; n=29) and Normal Glucose Tolerance (NGT) groups (n=32).
Results: Quantitative Coronary Angiography (QCA) showed no significant differences in EEM or vessel diameter between groups. However, the lumen CSA was significantly smaller in the DM group than in the NGT group. The plaque CSA and plaque burden were significantly greater in the DM and AGT groups than in the NGT group. Total insulin and glucose values and insulin and glucose values at 120 min after the OGTT correlated with plaque CSA; insulin values at 120 min after the OGTT showed the strongest correlation (R=0.505, P< 0.01).
Conclusions: Coronary plaque was identified among newly diagnosed DM or IGT patients even when the CAG appeared normal, suggesting that preventive measures against atherosclerosis should be initiated prior to DM development.
Keywords
Acute coronary syndrome, Abnormal glucose tolerance, Insulin, Intravascular ultrasound, Atherosclerosis, Diabetes mellitus
Introduction
Diabetes mellitus (DM) has been identified as a risk factor for cardiovascular disease. Mortality risk is 2–3 times greater in patients with DM than in those without [1,2]. It is well known that the prevalence of multivessel disease and long coronary lesions with small reference vessel diameters is high in patients with DM. Retrospective studies have demonstrated that patients with DM have more extensive coronary plaque compared with those without [3,4].
It is believed that atherosclerosis occurs in the pre-diabetes stage [5]. Therefore, abnormal glucose metabolism, such as that seen in patients with DM or impaired glucose tolerance (IGT), is recognized in over two-thirds of patients with cardiovascular disease such as acute myocardial infarction or angina pectoris [2,6,7].
Hyperglycemia, particularly postprandial hyperglycemia, is a well-known pre-diabetic condition and a risk factor for all-cause mortality and cardiovascular disease [1,8,9]. Some investigators have reported evidence that diabetic intervention therapy during the early phase of DM or IGT can have a preventive effect on cardiovascular events [10-12]. However, assessment of coronary plaque in the pre-diabetes stage has not been sufficiently investigated.
Coronary angiography (CAG) is the standard diagnostic test for coronary disease [13]. However, measurement of vessel walls and plaque is impossible using CAG because it only provides a two-dimensional (2D) image of the vessel lumen. Moreover, early atherosclerosis can sometimes be overlooked; furthermore, the assessment of diffuse disease is difficult. Intravascular ultrasound (IVUS) can measure the vessel wall and quantify the amount of coronary plaque, and it is commonly used as a diagnostic tool and an adjuvant treatment tool [14]. We examined the relationship between coronary atherosclerosis and abnormal glucose metabolism using IVUS in patients with acute coronary syndrome.
Methods
Subjects
IVUS-guided primary angioplasty of the Left Anterior Descending Artery (LAD) was performed for 205 patients with acute coronary syndrome between October 2003 and April 2007.
A normal coronary was defined by a %diameter stenosis (%DS) of < 20% according to Quantitative Coronary Angiography (QCA).
Patients with proximal LAD (n=66) and left main (n=17) coronary disease with a %DS of >20% were excluded from IVUS analysis.
Patients aged >80 years (n=15), patients with cardiogenic shock, and cases of death (n=10) and hemodialysis (n=1) were also excluded from the study. The remaining 96 patients were divided on the basis of DM status (Figure 1).
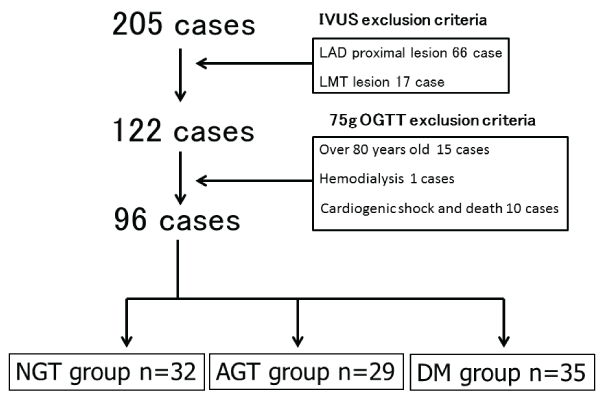
Figure 1: Enrollment criteria and study flow
DM (diabetes mellitus) group: DM was considered present if at least one of
the following criteria were present: 1) DM treatment, such as diet therapy,
medical therapy, or insulin use; 2) an HbA1c (National Glycohemoglobin
Standardization Program; NSGP) level of ≥6.5%; or 3) a Fasting Plasma
Glucose (FPG) level of ≥126 mg/dl. NGT (Normal Glucose Tolerance) group:
NGT was considered present if the FPG level was < 100 mg/dl and the 2h
plasma glucose (2h-PG) level was < 140 mg/dl following the 75-g oral glucose
tolerance test (OGTT). AGT (Abnormal Glucose Tolerance) group: Those
without DM and NGT.
View Figure 1
Patients were considered to have DM if they met at least one of the following criteria: 1) history of DM treatment, such as diet therapy, medical therapy, or insulin use; 2) an HbA1c (National Glycohemoglobin Standardization Program; NSGP) level of ≥6.5%, and/or 3) a fasting plasma glucose (FPG) level of >126 mg/dl.
Patients without DM underwent a 75-g oral glucose tolerance test (75-g OGTT) before discharge from the hospital and were divided into two groups according to the results.
Normal glucose tolerance (NGT) was defined as an FPG level of < 100 mg/dl and a 2h plasma glucose (2h-PG) level of < 140 mg/dl. Abnormal glucose tolerance (AGT) was defined as the absence of DM and NGT.
OGTT and blood sampling
Immediately after admission, baseline venous blood samples were obtained to measure ratios (%) of HbA1c (Japan Diabetes Society), total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and creatinine before any medical agents or other interventions were administered.
HbA1c was calculated as the National Glycohemoglobin Standardization Program (NGSP) equivalent value using the following formula: HbA1c (NGSP)%=1.02×HbA1c (Japan Diabetes Society; %) + 0.25% [15].
A baseline 75g OGTT was performed 2–3 weeks after the coronary intervention to avoid the possible influence of acute coronary syndrome on glucose tolerance. Blood samples were collected after a 12h overnight fast. A total of 75g of glucose was orally administered over a period of 5 min, and plasma glucose and insulin levels were measured before administration and 30, 60, and 120 min after administration (Figure 2).
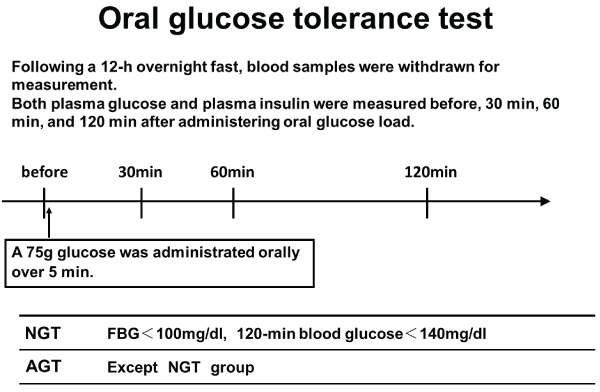
Figure 2: Oral glucose tolerance test (OGTT)
View Figure 2
IVUS and percutaneous coronary intervention
All patients received aspirin (162–200 mg) and intravenous heparin to achieve an activated clotting time of >250 s. Glycoprotein II b/III was not given to any patient. IVUS was performed with percutaneous coronary intervention and intracoronary administration of 125–250 μg of nitroglycerin. Data were acquired with commercially available IVUS transducers (30MHz/3.2F, Ultracross or 40 MHz/2.5F, Atlantis SR pro, Boston Scientific/SciMed, Natick, MA, USA). An imaging catheter was introduced into the mid-portion of the LAD and withdrawn to the left main by automatic pullback at a speed of 0.5 mm/s. Ultrasound images were recorded on S-VHS tape for offline analysis.
Quantitative IVUS and QCA analysis
Quantitative analyses of gray-scale IVUS images were performed according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on IVUS.16 Images of the site proximal to the LAD, 5 mm or more from a major branch such as the circumflex artery or major diagonal and diagnosed as normal on CAG, were evaluated for this study (Figure 3). The External Elastic Membrane (EEM) and lumen cross-sectional area (CSA) were measured. Plaque CSA was calculated as EEM minus lumen CSA, and plaque burden was calculated as plaque plus media CSA divided by EEM CSA.
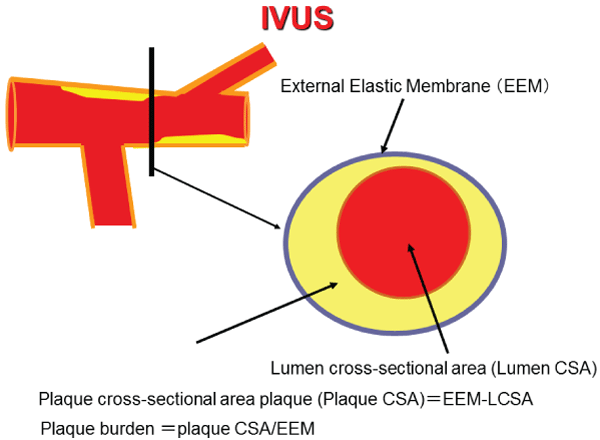
Figure 3: Cross-sectional intravascular ultrasound (IVUS) image of an
atherosclerotic human coronary artery and the measured position. The
plaque Cross-Sectional Area (CSA) was calculated as External Elastic
Membrane (EEM) minus lumen CSA, and plaque burden was calculated
as plaque plus media CSA divided by EEM CSA. Measured position: Left
Anterior Descending Artery (LAD) proximal to the lesion, 5mm or more from
a major branch such as the circumflex artery or major diagonal artery and
diagnosed as normal on coronary angiography (CAG).
View Figure 3
QCA, of the LAD proximal site assessed with IVUS, was performed with an automated edge detection system (CMS, Medis Medical Imaging Systems, Nuenen, The Netherlands) by experienced interventional cardiologists.
Statistical methods
Continuous variables are expressed as mean ± standard deviation (SD) and categorical variables as percentages. The chi-square test and Fisher’s exact test were used to compare frequency ratios between groups. Continuous variables were compared by analysis of variance (ANOVA). Differences between the mean 75g OGTT results were assessed by unpaired Student’s t-tests. Linear regression was used to determine whether plasma insulin and glucose levels correlated with plaque CSA. To evaluate associations between plaque CSA and other variables, stepwise forward multivariate linear regression analyses were performed. Candidate variables included all those parameters significant in univariate correlations. The entry criterion in the multivariate analyses was set at a significance level of p< 0.10.
A probability value of < 0.05 was considered statistically significant. All analyses were performed with SAS software version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient demographics
There were 35 patients with DM, 29 with AGT, and 32 with NGT (Table 1). The mean age was 65.8 ± 10.2, 62.4 ± 12.7, and 58.2 ± 13.0 years for patients with DM, AGT, and NGT, respectively; the DM patients were significantly older than the NGT patients (P< 0.05). There were no significant differences among the three groups with regard to body mass index, family history, or history of hypertension, dyslipidemia, smoking, prior myocardial infarction, and prior percutaneous coronary intervention.
![]()
Table 1: Baseline patient characteristics
View Table 1
Biochemical data are detailed in Table 2 and 3. There were no significant differences among the three groups with regard to total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, and serum creatinine levels. FPG values were 168 ± 63 mg/dl in the DM group, 94 ± 10 mg/dl in the AGT group, and 88 ± 7 mg/dl in the NGT group, while HbA1c values were 8.0% ± 1.4%, 5.7% ± 0.3%, and 5.5% ± 0.3%, respectively; both values were significantly higher in the DM group than in the other two groups (P< 0.05).
![]()
Table 2: Patient biochemical data
View Table 2
![]()
Table 3: Results of the 75g OGTT
View Table 3
QCA and IVUS findings
Vessel diameters on QCA were 3.3 ± 0.7mm, 3.2 ± 0.7mm, and3.4 ± 0.6mm in the DM, AGT, and NGT groups, respectively, while EEM values were 14.7 ± 3.9 mm2, 16.5 ± 4.2 mm2, and 14.9 ± 4.5 mm2, respectively; there were no significant differences among the three groups in both parameters. The lumen CSA measured 7.4 ± 2.8 mm2 in the DM group, 8.4 ± 2.3 mm2 in the AGT group, and 9.2 ± 3.3 mm2 in the NGT group, indicating that the lumen CSA was significantly smaller in the DM group than in the NGT group (P=0.0140; Figure 4A). The plaque CSA measured 7.3 ± 1.9 mm2, 8.1 ± 3.4 mm2, and 5.7 ± 2.6 mm2 (Figure 4B), while the plaque burden was 50.3% ± 9.4%, 47.9% ± 12.7%, and 38.3% ± 12.4% in the DM, AGT, and NGT groups, respectively (Figure 4C); plaque CSA and plaque burden were significantly greater in the DM and AGT groups than in the NGT group (P< 0.05), with no significant differences between the DM and AGT groups (Table 4).
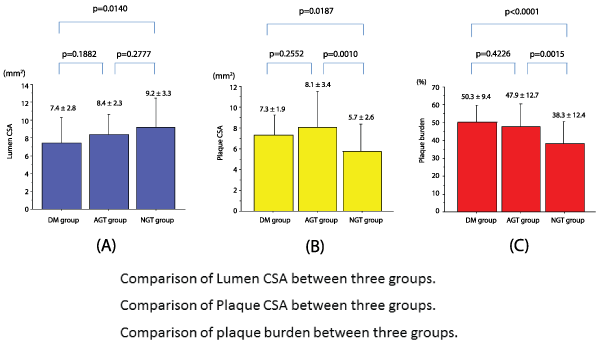
Figure 4: Intravascular ultrasound (IVUS) parameters
4A: Comparison of the lumen cross-sectional area (CSA) among the three groups. The lumen CSA was significantly smaller in the DM group than in the
NGT group (P=0.0140).
4B: Comparison of plaque CSA among the three groups. Plaque CSA was significantly greater in the DM and AGT groups than in the NGT group (P=0.0187,
P=0.0010, respectively). There was no significant difference between the DM and IGT groups.
4C: Comparison of plaque burden among the three groups. Plaque burden was significantly greater in the DM and AGT groups than in the NGT group
(P< 0.0001, P=0.0015, respectively). There was no significant difference between the DM and AGT groups.
View Figure 4
![]()
Table 4: QCA and IVUS data
View Table 4
Correlation of plaque CSA with insulin levels (fasting, 30 min, 60 min, 120 min, total), and glucose levels
The correlation of plaque CSA with insulin levels and glucose levels based on the 75g OGTT was evaluated. Insulin values at 120 min after the glucose challenge showed the strongest correlation with plaque CSA (R=0.505, P< 0.01; Figure 5A). Total insulin values after the glucose challenge (Figure 5B), glucose values at 120 min after the challenge (Figure 5C), and total glucose values after the challenge (Figure 5D) correlated with plaque CSA. Multivariable liner regression analysis found Insulin 120 min as the independent predictor of increasing Plaque CSA (Table 5).
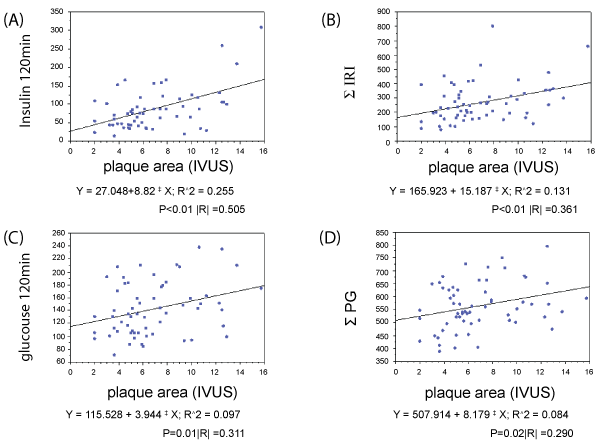
Figure 5: Correlations of insulin and glucose levels with the plaque cross-sectional area (CSA)
5A: Insulin levels at 120 min are highly correlated with the plaque CSA
5B: Glucose levels at 120 min and plaque CSA also correlate, albeit less strongly than insulin levels at 120 min and plaque CSA.
5C: Total immunoreactive insulin (SIRI) and plaque CSA show a significant but relatively weak correlation.
5D: Total plasma glucose (SPG) and plaque CSA show a significant but relatively weak correlation.
View Figure 5
![]()
Table 5: Results of Multivariable linear regression analysis
View Table 5
Discussion
This study evaluated IVUS findings and revealed that the plaque CSA was significantly greater in patients with AGT than in those with NGT, that there was no significant difference in the plaque CSA between patients with AGT and those with DM, and that insulin levels at 120 min after the 75-g OGTT correlated with plaque CSA in patients with AGT and NGT.
Atherosclerosis at the time of IGT and newly diagnosed DM
The most common cause of cardiovascular events represented by acute coronary syndrome is thrombus formation due to rupture or erosion of coronary artery plaque [17,18]. Existence of coronary plaque, regardless of the presence or absence of significant stenosis, is a risk factor for all-cause mortality and cardiovascular events [19,20]. It has been reported that DM has a strong relationship with the amount of coronary plaque as evaluated by IVUS or coronary computed tomography angiography [21-23] and that atherosclerosis initiates before DM development [5].
It has also been reported that carotid artery intimal and medial thickness is strongly correlated with postprandial glucose and is increased in the pre-diabetes stage [24,25]. These findings are consistent with the contention that atherosclerosis initiates before DM development, and they support the concept that atherosclerosis is not only a focal disease but also a systemic disease. Despite these findings, investigations of coronary artery plaque at various stages of IGT and the early phase of DM remain scarce. Therefore, we assessed coronary plaque in LAD proximal to the lesion, which appeared normal on CAG, using IVUS and revealed that the plaque CSA was significantly greater in the AGT group than in the NGT group and not significantly different in the DM group. These results strongly suggest that silent coronary atherosclerosis initiates and progresses during AGT.
Importance of coronary plaque during newly diagnosed DM and IGT
Many trials have demonstrated that the control of hypercholesterolemia [26-28] and hypertension [29,30] results in the prevention of cardiovascular events by control of cholesterol and blood pressure, regardless of the presence of DM. In contrast, intensive medical treatment targeted at HbA1c failed to demonstrate clinical benefits with regard to mortality [31-34].
However, it seems reasonable to expect that glucose control will contribute to the prevention of cardiovascular disease. In fact, meta-analysis of recent trials involving intensive DM control revealed that this strategy decreased the risk of myocardial infarction and cardiovascular death [35].
In addition, it was reported that diabetic intervention therapy at the stage of IGT and newly diagnosed DM is effective for the prevention of macrovascular disease [10-12,36]. Furthermore, establishment of diabetic intervention therapy at the time of DM diagnosis is useful for the prevention of macrovascular disease, and these effects have been documented to last for >10 years [37].
Our study demonstrated that coronary plaque in patients with AGT is similar to that in patients with DM and that insulin levels at 120 min after the glucose challenge were significantly associated with the quantity of coronary plaque. These findings strongly suggest the clinical benefits of glucose control in the initial stages of AGT. Furthermore, it may be reasonable to consider that the treatment of hyperinsulinemia during IGT or early-phase DM is essential for the prevention of macrovascular disease.
Study Limitations
This study had some limitations. First, it was a retrospective, single-center study; therefore, selection bias cannot be entirely ruled out. Second, cases of in-hospital death, cardiogenic shock, hemodialysis, and left main and LAD proximal lesions were excluded. These are usually severe cases, and most had DM. The possibility that these exclusions may have affected the results cannot be denied. We used 2D gray scale IVUS in this study; however, gray-scale IVUS has significant limitations with regard to the quality assessment of plaque [38].
Currently, the quantity of coronary artery plaque can be measured using three-dimensional IVUS or coronary computed tomography angiography, while the quality of plaque can be evaluated using coronary computed tomography angiography, virtual histology-IVUS, and iMap-IVUS [39-41].
Future studies should assess both the quality and quantity of plaque using the available technology.
Conclusions
Coronary plaque in patients with AGT was identified to be similar to that in patients with DM, and it was correlated with insulin levels at 120 min after a 75g OGTT. These results suggest that the progression of atherosclerosis occurs in the AGT phase and that the prevention of atherosclerosis should be given more careful consideration at the time of AGT or newly diagnosed DM.
Author Declaration
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
-
(1999) Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 354: 617-621.
-
Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, et al. (2007) Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 116: 151-157.
-
Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, et al. (1996) Coronary angioplasty in diabetic patients. The national heart, lung, and blood institute percutaneous transluminal coronary angioplasty registry. Circulation 94: 1818-1825.
-
Solymoss BC, Bourassa MG, Campeau L, Sniderman A, Marcil M, et al. (2004) Effect of increasing metabolic syndrome score on atherosclerotic risk profile and coronary artery disease angiographic severity. Am J Cardiol 93: 159-164.
-
Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK (1990) Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 263: 2893-2898.
-
Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, et al. (2004) The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J 25: 1880-1890.
-
Ramachandran A, Chamukuttan S, Immaneni S, Shanmugam RM, Vishnu N, et al. (2005) High incidence of glucose intolerance in Asian-Indian subjects with acute coronary syndrome. Diabetes Care 28: 2492-2496.
-
Balkau B, Shipley M, Jarrett RJ, Pyorala K, Pyorala M, et al. (1998) High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the whitehall study, the paris prospective study, and the helsinki policemen study. Diabetes Care 21: 360-367.
-
Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22: 233-240.
-
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, et al. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343-1350.
-
Li G, Zhang P, Wang J, Gregg EW, Yang W, et al. (2008) The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371: 1783-1789.
-
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, et al. (2003) Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the stop-niddm trial. JAMA 290: 486-494.
-
Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ (1987) Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 316: 1371-1375.
-
Nissen SE, Yock P (2001) Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 103: 604-616.
-
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, et al. (2012) Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes S. International clinical harmonization of glycated hemoglobin in japan: From japan diabetes society to national glycohemoglobin standardization program values. J Diabetes Investig 3: 39-40.
-
Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, et al. (2001) American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus). A report of the american college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 37: 1478-1492.
-
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92: 657-671.
-
Shah PK (1998) Role of inflammation and metalloproteinases in plaque disruption and thrombosis. Vasc Med 3: 199-206.
-
Ricciardi MJ, Meyers S, Choi K, Pang JL, Goodreau L, et al. (2003) Angiographically silent left main disease detected by intravascular ultrasound: a marker for future adverse cardiac events. Am Heart J 146: 507-512.
-
Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, et al. (2011) Mortality risk in symptomatic patients with nonobstructive coronary artery disease: A prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 58: 510-519.
-
Kaneda H, Kataoka T, Ako J, Honda Y, Yock PG, et al. (2008) Coronary risk factors and coronary atheroma burden at severely narrowing segments. Int J Cardiol 124: 124-126.
-
Nicholls SJ, Tuzcu EM, Crowe T, Sipahi I, Schoenhagen P, et al. (2006) Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. J Am Coll Cardiol 47: 1967-1975.
-
Zheng M, Choi SY, Tahk SJ, Lim HS, Yang HM, et al. (2011) The relationship between volumetric plaque components and classical cardiovascular risk factors and the metabolic syndrome a 3-vessel coronary artery virtual histology-intravascular ultrasound analysis. JACC Cardiovasc Interv 4: 503-510.
-
Bonora E, Kiechl S, Oberhollenzer F, Egger G, Bonadonna RC, et al. (2000) Impaired glucose tolerance, Type II diabetes mellitus and carotid atherosclerosis: prospective results from the Bruneck Study. Diabetologia 43: 156-164.
-
Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, et al. (2000) Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 23: 1830-1834.
-
Goldberg RB, Mellies MJ, Sacks FM, Moyé LA, Howard BV, et al. (1998) Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: Subgroup analyses in the cholesterol and recurrent events (care) trial. The care investigators. Circulation 98: 2513-2519.
-
Pyorälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G (1997) Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the scandinavian simvastatin survival study (4s). Diabetes Care 20: 614-620.
-
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, et al. (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (cards): Multicentre randomised placebo-controlled trial. Lancet 364: 685-696.
-
Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, et al. (2000) Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321: 412-419.
-
(1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317: 703-713.
-
(1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837-853.
-
Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545-2559.
-
ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560-2572.
-
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, et al. (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129-139.
-
Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, et al. (2009) Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med 151: 394-403.
-
Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, et al. (2009) Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 58: 2642-2648.
-
Holman RR1, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577-1589.
-
Di Mario C, Görge G, Peters R, Kearney P, Pinto F, et al. (1998) Clinical application and image interpretation in intracoronary ultrasound. Study group on intracoronary imaging of the working group of coronary circulation and of the subgroup on intravascular ultrasound of the working group of echocardiography of the european society of cardiology. Eur Heart J 19: 207-229.
-
Marso SP, Mercado N, Maehara A, Weisz G, Mintz GS, et al. (2012) Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging 5: S42-52.
-
Araki T, Nakamura M, Utsunomiya M, Sugi K (2012) Visualization of coronary plaque in type 2 diabetes mellitus patients using a new 40 MHz intravascular ultrasound imaging system. J Cardiol 59: 42-49.
-
Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, et al. (2006) Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol 47: 2405-2412.





