TSH is a peptide hormone synthetized and secreted by pituitary with fundamental importance for thyroid function. TSH receptors are found in other tissue than thyroid playing diverse roles, including erythrocyte. Based on the fact that deformability is one of the most important features of a mature erythrocyte, we aimed to verify if TSH modulates erythrocytes volume and, if does, which signaling is involved in this modulation. Using hemolysis assay, we create a hemolysis curve of erythrocyte in presence of TSH in different concentrations (1, 2, 3 and 5 mIU/L). By adding AICAR, Wortmannin and Akt 1/2 inhibitor, it was possible to observe whether these pathways had an influence on TSH-reduced hemolysis in specific NaCl concentration of 0.4%. Changing concentrations of NaCl from 0.1 to 0.9% was accompanied by marked hemolysis in very low sodium chloride concentrations of 0.1, 0.2, 0.3 and 0.4% and a substantial decrease in hemolysis in 0.5 to 0.9% of NaCl occurred in control treatment. After TSH treatment, all concentrations tested were able to reduce significantly hemolysis in 0.4% NaCl. AICAR, Wortmannin and Akt 1/2 inhibitor reversed the effect of TSH in all concentrations. In conclusion, we observed that TSH ranging from 1 to 5 mIU/L improves RBC resistance to hemolysis and this effect is made by inhibiting AMPK-dependent pathway and concomitant activation of PI3K/Akt signaling pathway.
TSH, Erythrocyte, Osmotic fragility, Signaling pathway
Thyroid-stimulating hormone (TSH) is a peptide hormone synthetized and secreted by pituitary with fundamental importance for thyroid function and balance, affecting iodine uptake, thyroxine and triiodothyronine production and, as a consequence, whole body metabolism [1,2]. It acts through thyroid-stimulating hormone receptor (TSHr), a classical seven transmembrane G-protein coupled receptor [3], being usually coupled to Gαs, increasing cAMP production and activation of PKA or even to Gαq and either activating Ras/Raf/Mek/Erk or PI3K/Akt signaling pathways [4].
TSH receptors are found in other tissue than thyroid playing diverse roles. In brown adipose tissue it was demonstrated that TSH increase lipolysis, inhibit leptin mRNA and increase O2 consumption with Erk and Akt phosphorylation [5] and also acts as a survival factor via PI3K/Akt in 3T3-L1 preadipocytes cell model [6]. mRNA and protein expression of TSHr was detected by Crisanti, et al., in primary cultured human astrocytes besides neuronal cells [7]; TSH also was able to increase hepatic gluconeogenesis via cAMP/PKA pathway [8]. In the same way, Nicolini and co-workers showed that erythrocyte of patients with subclinical hypothyroidism has increased number of ouabain-binding sites and latter ratified the presence of TSHr in these cells with co-immunoprecipitation [9,10]. These data points to the paradigm of functional TSHr in erythrocytes and remarks a possible role of hormone actions in nonthyroidal cells.
Erythrocytes are cells responsible mainly for gas exchange process, so it is necessary a high degree of deformability in order to facilitate their passage through reduced caliber vessels and thus oxygenate tissues [11]. This deformability is conferred by several factors such as a high area-volume ratio (conferred by the biconcave disc shape) and the degree of fluidity of the plasma membrane [12]. Thus, deformability is one of the most important features of a mature erythrocyte, with several factors affecting it. PI3K/Akt signaling pathway in erythrocytes along with nitric oxide synthetase showed to improve deformability [12]. Additionally, Rho Kinase plays an important role in erythrocyte deformability when a specific inhibitor, Y-27632, increased erythrocyte deformability by inducing ATP release from these cells [13].
Red blood cells (RBC) deformability is a product of hydration, cytoskeletal proteins and structure, metabolism, vesiculation, adaptive responses and hemoglobin content with numerous techniques to measure directly or indirectly this feature like osmotic fragility test (OFT) [14]. OFT is often performed to help diagnosis of hereditary hemolytic diseases [15], based on the fact that controlling intracellular volume in a normal cell requires activation of diverse signaling pathways and transmembrane enzymes that control ion flux across the cell [16,17]. Therefore, the question that lead this work was whether TSH modulates erythrocytes volume regulation and, if does, which signaling is involved in this modulation.
Collection of human blood samples for this study was conducted according to the protocols approved by the Research Ethics Committee of the Federal University of Rio de Janeiro (Protocol 2.889.952) and the project is registered in Brazil Platform under the CAAE number 88140418.5.0000.5699. The samples analyzed in this study were obtained from men and women and everyone was informed about the study and those who agreed to participate completed the written informed consent form for the collection of samples and subsequent use. Those with any hemoglobinopathies and those taking controlled medication were excluded. Blood samples were collected in anticoagulant tubes with EDTA by puncture in antecubital fossa.
Blood was collected in 4 mL EDTA tubes. These tubes were centrifuged for 10 minutes at approximately 3,000 rpm. After this, the plasma and buffy coat was removed and discarded. The RBC was washed with PBS (phosphate buffered saline) three times (tubes were homogenized by inversion and centrifuged in the same parameters as above). Wide range of NaCl concentrations (0.1%-0.9%) was added, followed by TSH in four concentrations (1, 2, 3 and 5 mIU/L), and RBC, resulting in an experiment with a final volume of 300 µl and 1% hematocrit. The tubes were placed in the water bath, 37 ℃, and all experiments was incubated for one hour with hormone whether combined or not with inhibitors or activators, centrifuged for 10 minutes, 10,000 rpm and the supernatant were carefully removed and pipetted (200 µl) into the 96-well plate. The reading was made in an Elisa reader (Tecan GENios®) at 540 nm absorbance.
The same procedure of common hemolysis was performed, however, 5-Aminoimidazole-4-carboxamide 1-β-D- ribofuranoside, Acadesine, N1-(β-D-Ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR), wortmannin and Akt 1/2 inhibitor (1,3-Dihydro-1-(1-((4-(6-phenyl-1H- imidazo[4,5-g]quinoxalin-7-yl)phenyl)methyl)-4-piperidinyl)-2H-benzimidazol-2-one trifluoroacetate salt hydrate) was added. The amount of NaCl was recalculated to maintain the final volume of 300 βl and 1% hematocrit and next experiments followed the same methodology as the previously mentioned Hemolysis; however, they were only performed with the concentration of 0.4% NaCl. 0.1% NaCl was used as a 100% hemolysis control, without adding any substance.
GraphPad Prism 5 was used to plot the graphs and to perform statistical analysis, with all data expressed as Mean ± SEM. For all experiments, an ANOVA with Tukey's post-test was used for analysis of the significant differences (*for p < 0.05, **for p < 0.01 and ***for p < 0.001, when compared to control and # for p < 0.05, ## for p < 0.01 and ### for p < 0.001, when compared to same TSH concentration without signaling pathway modulators) with a confidence interval of 95%.
Changing concentrations of NaCl from 0.1 to 0.9% was accompanied by marked hemolysis in very low sodium chloride concentrations of 0.1, 0.2, 0.3 and 0.4%. We took 0.1% as 100% of hemolysis and compared the amount of subsequent hemolysis that ranged around 100% in low sodium chloride concentrations control curves showed in (Figure 1A, Figure 1B, Figure 1C and Figure 1D), where 0.2% NaCl gives 96.58%, 0.3% gives 108.59% and 0.4% gives 98.12% of hemolysis in control curve. There is a substantial decrease in hemolysis in 0.5 to 0.9% of NaCl in control condition of 10.58%, 10.79%, 9.29%, 9.01% and 8.37% of hemolysis respectively. When TSH 1 mIU/L was added to the media (Figure 1A), there was a left shift of the curve with statistically decreased hemolysis in 0.3% and 0.4% of NaCl (89.75% and 67.13%, respectively) compared to control curve. TSH 2 mIU/L (Figure 1B) exhibited a 79.67% of hemolysis in NaCl 0.4% and higher concentrations of the hormone followed the same pattern with decreased hemolysis in the same 0.4% NaCl concentration (78.33% for 3 mIU/L of TSH in Figure 1C and 84.88% for 5 mIU/L in Figure 1D).
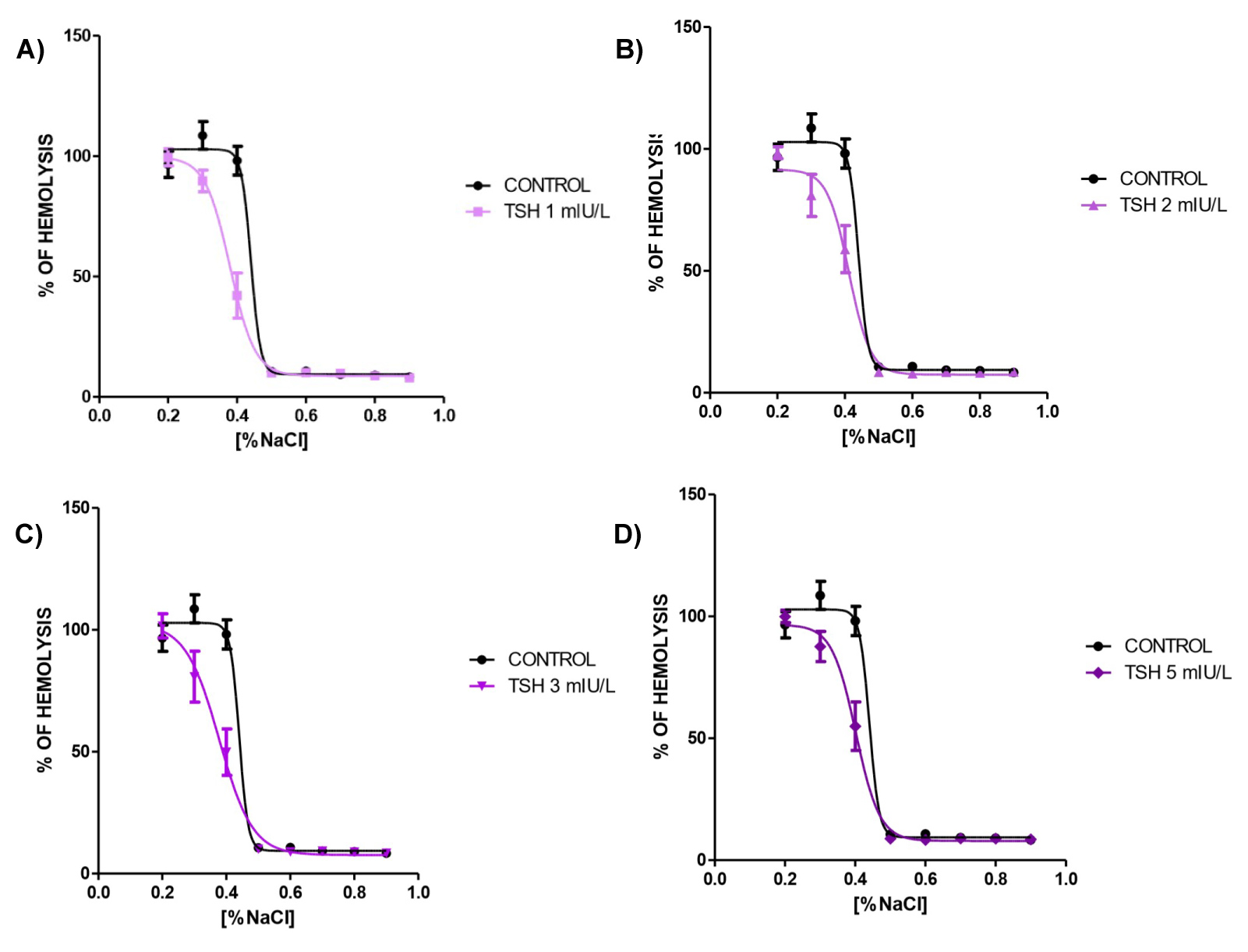 Figure 1: A, B, C, D, Osmotic fragility curve of human blood treated with TSH 1, 2, 3 and 5 mIU/L for one hour, respectively. N = 5 and all experiments was performed in triplicate.
View Figure 1
Figure 1: A, B, C, D, Osmotic fragility curve of human blood treated with TSH 1, 2, 3 and 5 mIU/L for one hour, respectively. N = 5 and all experiments was performed in triplicate.
View Figure 1
Interestingly, in NaCl 0.4% there was a statistically significantly reduce in hemolysis in all TSH concentrations (Figure 2) pointing that the hormone act reducing osmotic fragility in osmotic stressed erythrocytes.
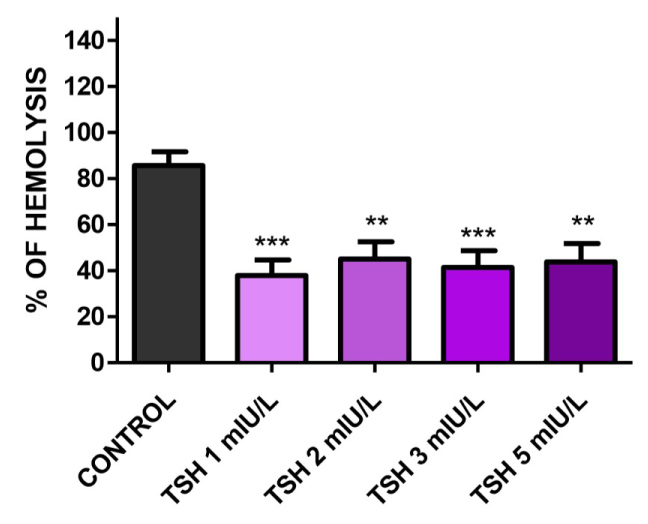 Figure 2: Osmotic fragility in NaCl 0.4% treated with TSH 1, 2, 3, and 5 mIU/L for one hour. There was decrease in hemolysis in all TSH concentrations: 37.9675%, 45.0328%, 41.4301% and 43.8512%, respectively. N = 7 and all experiments was performed in triplicate. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control.
View Figure 2
Figure 2: Osmotic fragility in NaCl 0.4% treated with TSH 1, 2, 3, and 5 mIU/L for one hour. There was decrease in hemolysis in all TSH concentrations: 37.9675%, 45.0328%, 41.4301% and 43.8512%, respectively. N = 7 and all experiments was performed in triplicate. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control.
View Figure 2
Adding AMPK activator 1 mM AICAR to NaCl 0.4% media lead us to verify the influence of this pathway in TSH-reduced hemolysis. As shown in Figure 3, AICAR blunted the effect of TSH in all concentrations studied, with 1, 2, 3 and 5 mIU/L displaying 91.96%, 92.21%, 86.14% and 86.76% of hemolysis, respectively. This was statistically significant when compared to control and when each concentration after AICAR introduction was compared to the same concentration without AICAR.
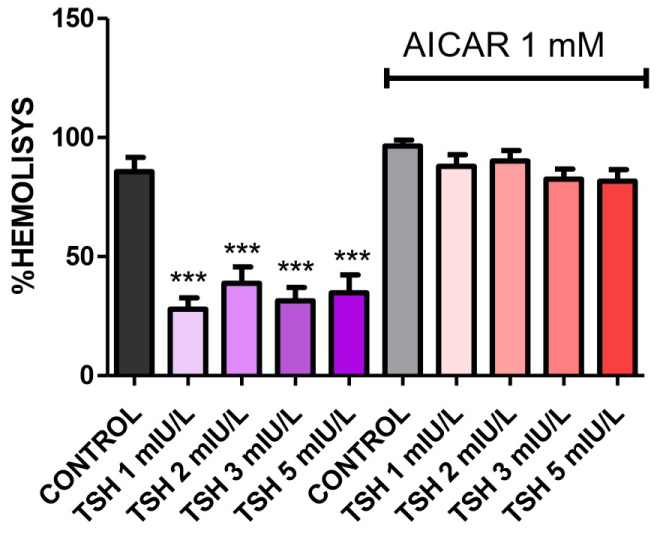 Figure 3: Osmotic fragility in NaCl 0.4% treated with AICAR plus TSH 1, 2, 3, and 5 mIU/L for one hour. AICAR reversed the effect of TSH in all concentrations: 91.96%, 92.21%, 86.14% and 86.76%, respectively. N = 4. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. same TSH concentration not treated with AICAR.
View Figure 3
Figure 3: Osmotic fragility in NaCl 0.4% treated with AICAR plus TSH 1, 2, 3, and 5 mIU/L for one hour. AICAR reversed the effect of TSH in all concentrations: 91.96%, 92.21%, 86.14% and 86.76%, respectively. N = 4. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. same TSH concentration not treated with AICAR.
View Figure 3
PI3K inhibitor wortmannin in 10 µM was used to assess the involvement of PI3K activation. As shown in Figure 4, wortmannin reversed the effects of TSH in all concentrations with 81.73%, 78.41%, 75.80% and 78.64% for 1, 2, 3 and 5 mIU/L respectively with statistically difference when compared to control and when compared to paired concentrations of TSH.
 Figure 4: Osmotic fragility in NaCl 0.4% treated with Wortmannin plus TSH 1, 2, 3,and 5 mIU/L for one hour. All concentrations had the effect of TSH reversed by Wortmannin (81.73%, 78.41%, 75.80% and 78.64%, respectively) when compared to control and when compared paired TSH observations. N = 3 and all experiments was performed in triplicate. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. same TSH concentration not treated with wortmannin.
View Figure 4
Figure 4: Osmotic fragility in NaCl 0.4% treated with Wortmannin plus TSH 1, 2, 3,and 5 mIU/L for one hour. All concentrations had the effect of TSH reversed by Wortmannin (81.73%, 78.41%, 75.80% and 78.64%, respectively) when compared to control and when compared paired TSH observations. N = 3 and all experiments was performed in triplicate. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. same TSH concentration not treated with wortmannin.
View Figure 4
As for possible involvement of Akt pathway, we also measured hemolysis with Akt 1/2 inhibitor in 1 mM concentration as displayed in Figure 5. In the same way seen in previous experiments, use of the inhibitor reversed the observed diminished hemolysis when compared to control, with 84.21%, 85.78%, 83.36% and 86.57% amount of hemolysis and statistical difference compared between TSH concentrations too.
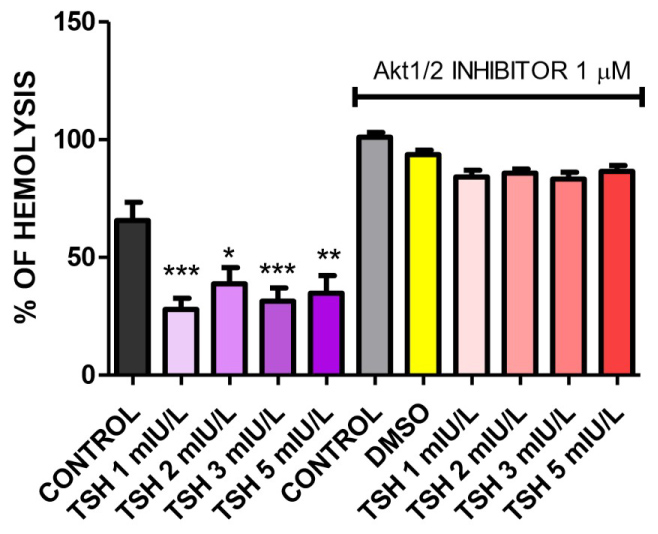 Figure 5: Osmotic fragility in NaCl 0.4% treated with Akt1/2 inhibitor plus TSH 1, 2, 3, and 5 mIU/L for one hour. The inhibitor reversed the observed diminished hemolysis when compared to control, with 84.21%, 85.78%, 83.36 and 86.57% respectively. N = 3 and all experiments was performed in triplicate. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. same TSH concentration not treated with Akt 1/2 inhibitor.
View Figure 5
Figure 5: Osmotic fragility in NaCl 0.4% treated with Akt1/2 inhibitor plus TSH 1, 2, 3, and 5 mIU/L for one hour. The inhibitor reversed the observed diminished hemolysis when compared to control, with 84.21%, 85.78%, 83.36 and 86.57% respectively. N = 3 and all experiments was performed in triplicate. Values are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. same TSH concentration not treated with Akt 1/2 inhibitor.
View Figure 5
Erythrocyte volume control is essential for its proper function and, therefore, for life: Overhydration or hypohydration are common fate of diseases involving primarily erythrocytes as seen in plasmodium infection, alcohol intoxication, sickle cell disease, hereditary spherocytosis and thalassemia [18-21]. Considering this, although lack of organelles and nuclei, erythrocytes access sophisticated ways to control ion flux. Na+/K+-ATPase is one of the key modulators of cell volume with recommendation to be one of the predictors to disease diagnosis and by being modulated by hormones and xenobiotics [22-25]. Huisjes in an elegant review exposed the importance of not only Na+/K+-ATPase but calcium pumps (PCMA I and PCMA I4), Na+-K+-2Cl- cotransporter, K+-Cl- cotransporter, Na+/H+ exchanger, Cl--HCO3- exchanger, Gardos channel, and others transporters [14]. Also, in the same review, special attention was payed to methods and techniques for measuring red blood cell deformability, being one of them, even with disadvantages as all methods, the osmotic fragility test [14].
In this study, we assessed the effects of TSH in controlling red cell volume using the simple OFT technique. There was a significant reduction in hemolysis in RBC treated with TSH 1, 2, 3 and 5 mIU/L in 0.4% NaCl, showing that TSH is able to protect lysis even in a very hostile media. Although literature presents an increase in osmotic fragility when animal models are subjected to experimental (drug-induced) hypothyroidism, our data shows that TSH levels are positively correlated with erythrocyte survival under hypotonic conditions in an isolated system [26,27]. This can be due to adverse experimental conditions of animal model with also reported by these authors increased oxidative stress. Nevertheless, is important to highlight that erythrocytes employed in this study was from euthyroid individuals, which might be different from hypothyroid erythrocytes.
TSH is classically associated to cAMP/PKA and PLC/PKC signaling pathway [28] and others studies points to alternative pathways like activation of ERK 1/2 and NFκB [29]. In an attempt to elucidate possible signaling pathways that can be trigged by TSH in this specific system, we added AICAR, an AMPK activator, to assess whether the late is involved in TSH- mediated effects. We notice that AICAR blunted the protection in all concentrations studied, indicating that AMPK activity is suppressed by TSH treatment. This is in accordance with literature when Shudong, et al. reported that AICAR blocks the TSH- induced expression of specific genes in liver [30]. Besides, Thali and co- workers reported the presence of AMPK in red blood cell lysates [31] and AMPK-deficient mice exhibited an increased hemolysis resistance around 150 mOsmol/L (correspondent to 0.4% NaCl) when compared to wild-type control model [32], ratifying our findings.
In order to continue searching for other proteins modulated by TSH, we assessed PI3K involvement by adding wortmannin, a specific inhibitor of this kinase. Our result showed that wortmannin also reverted the protection promoted by TSH in all concentrations studied, revealing the link between TSH and PI3K in erythrocytes. Jafari, et al. demonstrated the importance of PI3K in differentiation and maturation of RBC [33]; insulin can also activates this pathway in order to prevent ATP release from erythrocytes [34]. It was also reported that pharmacological inhibition of PI3K pathway led to reduced erythrocyte deformability through reduced NO production, confirming our results that shows increased osmotic resistance and indirect increase in deformability [14,35].
As a sequence of PI3K pathway, we assayed the activation of Akt in this system and confirmed, as expected, that use of Akt 1/2 inhibitor recoil the protection seen with TSH in all concentrations. Impaired erythrocyte deformability by O2- was reported to be due to impaired Akt activation [12] as well as NO Activation upstream Akt kinase improved deformability of erythrocytes, also highlighting the data found in the present work.
Together, our data points that TSH in 1 to 5 mIU/L improves RBC resistance to hemolysis, as an indirect measure of deformability, and this effect is made by inhibiting AMPK-dependent pathway and concomitant activation of PI3K/Akt signaling pathway, as show in Figure 6.
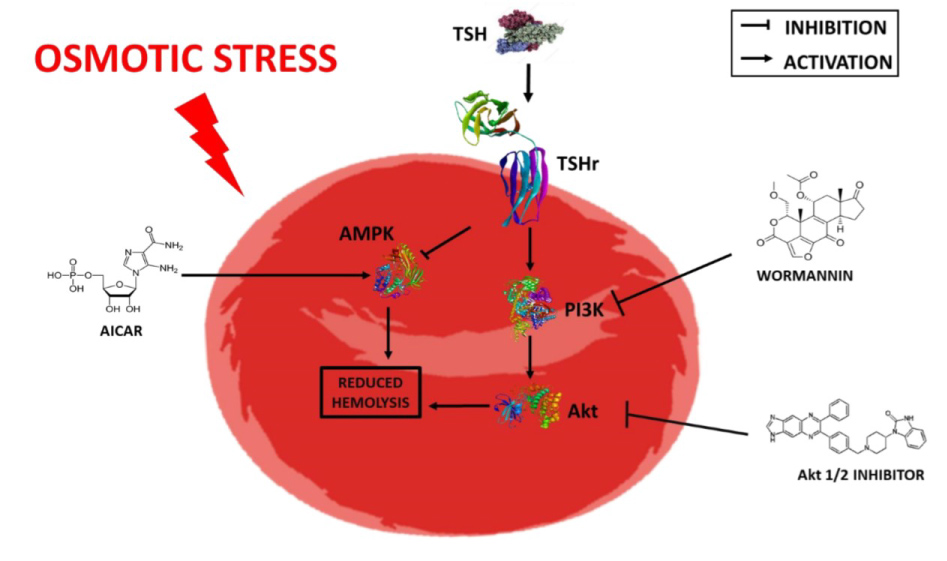 Figure 6: TSH ameliorates erythrocyte resistance to hemolysis. When subjected to an osmotic stress situation, TSH binds to its receptor (TSHr), on the erythrocyte membrane, inhibiting AMPK-dependentpathway (stimulatedby AICAR) and concomitantly activates the PI3K (inhibited by wortmannin)/Akt (inhibited by Akt ½ inhibitor) signaling pathway.
View Figure 6
Figure 6: TSH ameliorates erythrocyte resistance to hemolysis. When subjected to an osmotic stress situation, TSH binds to its receptor (TSHr), on the erythrocyte membrane, inhibiting AMPK-dependentpathway (stimulatedby AICAR) and concomitantly activates the PI3K (inhibited by wortmannin)/Akt (inhibited by Akt ½ inhibitor) signaling pathway.
View Figure 6
There is no conflict of interest.
We thank FAPERJ, FUNEMAC, CAPEs and CNPq for financial support.
This study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (JCNE-FAPERJ, E-26/201.520/2014; Edital Sediadas- FAPERJ, E-26/010.000175/2016), Conselho Nacional de Desenvolvimento Científico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Ciências sem Fronteiras/PVE/88881.062218/2014-0), who supported the scholarship.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No competing financial interests exist.
Each author contributed in equal parts for this manuscript.