International Journal of Blood Research and Disorders
In vitro Influence of Fibrinogen and Factor XIII on Viscoelastic Characteristics of Clot Formation under Hypothermia and Acidosis
DA Ammon1, H Andruszkow2, L Sieg1, M Wilhelmi3, CF Weber4, N Rahe-Meyer1,5 and AA Hanke1*
1Department of Anaesthesiology and Intensive Care Medicine, Hannover Medical School, Germany
2Department of Trauma and Reconstructive Surgery, Universitiy Hospital Aachen, Aachen, Germany
3Department of Trauma and Reconstructive Surgery, Hannover Medical School, Germany
4Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Germany
5Department of Anaesthesiology, St. Franziskus Hospital Bielefeld, Bielefeld, Germany
*Corresponding author:
PD Dr. AA Hanke, Department of Anaesthesiology and Intensive Care Medicine, Hannover Medical School, Carl-Neuberg-Strasse 1, D-30625 Hannover, Germany, E-mail: hanke.alexander@mh-hannover.de
Int J Blood Res Disord, IJBRD-2-019, (Volume 2, Issue 2), Research Article; ISSN: 2469-5696
Received: October 29, 2015 | Accepted: November 25, 2015 | Published: November 27, 2015
Citation: Ammon DA, Andruszkow H, Sieg L, Wilhelmi M, Weber CF, et al. (2015) In vitro Influence of Fibrinogen and Factor XIII on Viscoelastic Characteristics of Clot Formation under Hypothermia and Acidosis . Int J Blood Res Disord 2:019. 10.23937/2469-5696/1410019
Copyright: © 2015 Ammon DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Hypothermia and acidosis are risk factors for coagulopathy during trauma. We investigated influence of fibrinogen and factor XIII on whole blood coagulation under hypothermia and acidosis in an In vitro model.
Methods: Ten healthy volunteers were included. Rotation Thromboelastometry was performed at 37°C and 32°C at pH 7.4, 7.2 and 7.0. Samples were subdivided in four treatment groups adding isotonic saline for dilution compensation, fibrinogen, factor XIII, or both substrates. Baseline was no treatment at 37°C and normal pH. Samples were incubated for 30 minutes and measured after extrinsically activation and fibrin polymerization was tested.
Results: Fibrinogen, but not FXIII improved coagulation significantly. Furthermore, combined administration of fibrinogen and FXIII did not show differences to administration of fibrinogen alone.
Conclusion: According to our findings application of FXIII under hypothermia and acidosis seems ineffective, at least when FXIII levels are within normal range. A possible beneficial effect is conceivable with reduced FXIII levels. Further research is required.
Introduction
Acute coagulopathy in trauma patients is generally associated with high mortality [1]. Though decreasing during the last years, exsanguination is, beyond traumatic brain injury, the main cause of death in multiple trauma patients in the early onset [2,3]. Hypothermia and acidosis occur in patients suffering from multiple injuries and independently induce coagulopathy [4]. While acidosis alone severely impairs plasmatic coagulation, platelet function and coagulation time is more affected in presence of hypothermia [5]. Beside a decreased enzymatic function of coagulant factors, another cause for coagulation impairment in different settings is loss of platelets and coagulant proteins such as fibrinogen. Furthermore, fibrinogen is affected by dilutional coagulopathy. It is the first coagulation factor that decreases in trauma associated dilution coagulopathy [6,7]. Intensified blood loss during coagulopathy can occur when fibrinogen deficiency is combined with reduced levels of FXIII, a transglutaminase that improves clot firmness by crosslinking of fibrin monomers [8,9]. Previous studies showed an improvement of platelet function as well as overall clot firmness during hypothermia and acidosis by administration of fibrinogen [10,11] and suggested a higher survival rate in an animal model [12]. An effect of additional administration of FXIII under such circumstances has not been examined to date. Thus we tested fibrinogen and FXIII in a model of hypothermia and acidosis on viscoelastic effects on clot formation In vitro as assessed by rotation thromboelastometry.
Materials and Methods
The study was approved by the local ethic committee and was conducted according to the Helsinki Declarations and European Unions Convention on Human Rights and Biomedicine.
10 healthy volunteers were included into the study after oral and written informed consent. All volunteers were free of coagulation affecting medication or bleeding disorders.
Blood samples were drawn from a cubital vein using an 18 gauge cannula. The first 5 ml were discarded. 21 ml were collected into 7 citrated tubes (Sarstedt AG & Co, Numbrecht, Germany). Fibrinogen and FXIII levels were determined in the local standard laboratory (Fibrinogen: Dade® Thrombin Reagent FXIII: Berichrom® FXIII; both Siemens Healthcare Diagnostics, Marburg, Germany; both measured on a SYSMEX CS-2100i, SYSMEX GmbH, Norderstedt, Germany). Samples for rotation thromboelastometry were prepared according to a protocol which is displayed in table 1.
![]()
Table 1: Sample preparation protocol. Groups are: ISO (isotonic saline for compensation of dilutional effects), FIB+ (fibrinogen), FXIII+ (factor XIII), FIB+FXIII+ (both substrates).
View Table 1
Technical facts of rotation thromboelastometry (ROTEM®, Tem International GmbH, Munich, Germany) have been described elsewhere [13], but will be repeated briefly in the following. Rotation thromboelastometry acts by using a pin which is dipped into a blood-filled cup. The pin is rotating in a precisely defined angle. While the blood begins to clot fibrin polymers aggravate rotation which is charted on a diagram, which in this way enables the visualization of blood coagulation. All measurements were conducted due to manufacturer's instructions. We performed two ROTEM analyses: EXTEM and FIBTEM, both as extrinsically activated tests (activation by recombinant tissue factor). Additionally, FIBTEM contains Cytochalasin D which inhibits platelet function and displays fibrin polymerization only [14]. Following EXTEM parameters were recorded: coagulation time (CT [s]) which describes the time from the beginning of the reaction until first clot formation; clot formation time (CFT [s]): time from first clot formation to a defined clot firmness (20 mm); clot firmness after 20 minutes (A20 [mm]) as a marker for the clot stability. Regarding FIBTEM A20 values were recorded.
Sample preparation
Samples were subdivided into four groups according to addition of different substrates:
• Native (ISO: addition of 300 mcl 0.9% sodium chloride for compensation of dilutional effects)
• Fibrinogen (FIB+: 0.25 mg fibrinogen in 300 mcl, equivalent to 4 g fibrinogen (Haemocompletan, CSL Behring, Hattersheim, Germany) in a 70 kg human)
• FXIII (FXIII+: 0.2 IE FXIII (Fibrogammin, CSL Behring, Hattersheim, Germany) in 300 mcl, equivalent to 2500 IE FXIII in a 70 kg human)
• Fibrinogen and FXIII in above mentioned concentrations (FIB+FXIII+)
Measuring points were:
1. 37°C, pH 7.4 (marked as baseline)
2. 32°C, pH 7.4
3. 32°C, pH ≤ 7.2 ( + 45 μl 0.5 mol/L hydrochloric acid [HCL])
4. 32°C, pH ≤ 7.0 ( + 100 μl 1 mol/L hydrochloric acid [HCL])
5. Resulting pH of each essay was controlled by an automated blood as analyzer (ABL FLEX-900, Radiometer, and Copenhagen, Denmark).
For hypothermic measurements the ROTEM device was cooled down by the integrated soft- and hardware. The regular device integrated thermal control was used to reassure accurate temperature for the specific measurements.
Statistical analysis
The data were analyzed using the statistical package for the social sciences (SPSS; version 20; IBM Inc., Somers, NY, USA). Incidences are presented with counts or percentages while continuous values are presented as mean ± standard deviation (SD). Differences between the groups were evaluated with analysis of variance (ANOVA) for continuous data. The Tukey post-hoc test was used when appropriate to identify differences between the aforementioned classified groups.
A two sided p-value < 0.05 was considered to be significant.
Results
Blood samples were withdrawn from seven males and three females, ages 29 ± 6 years. ROTEM results are shown in figure 1, figure 2, figure 3 and figure 4.
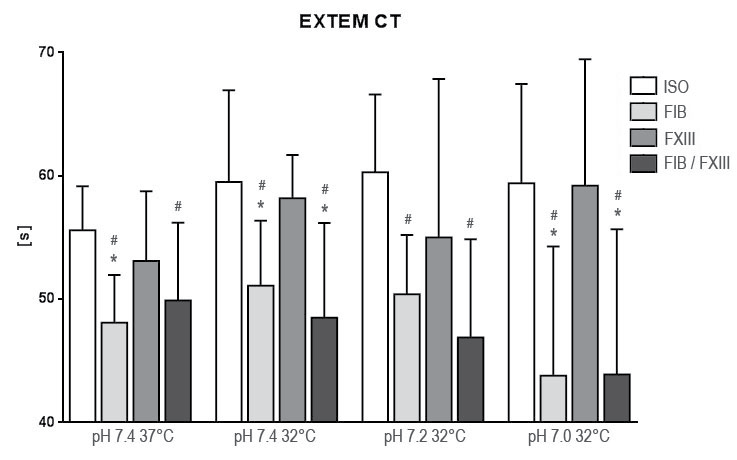
.
Figure 1: Results of rotation thrombelastometry coagulation time (CT) after extrinsic activation under hypothermia and acidosis. Groups are Native: control group with addition of isotonic saline solution for compensation of dilutional effects; Fib: addition of Fibrinogen; FXIII: addition of Factor XIII; Fib FXIII: addition of both substances. Asterisks (*) mark significant differences to ISO, # mark significant differences to FXIII.
View Figure 1
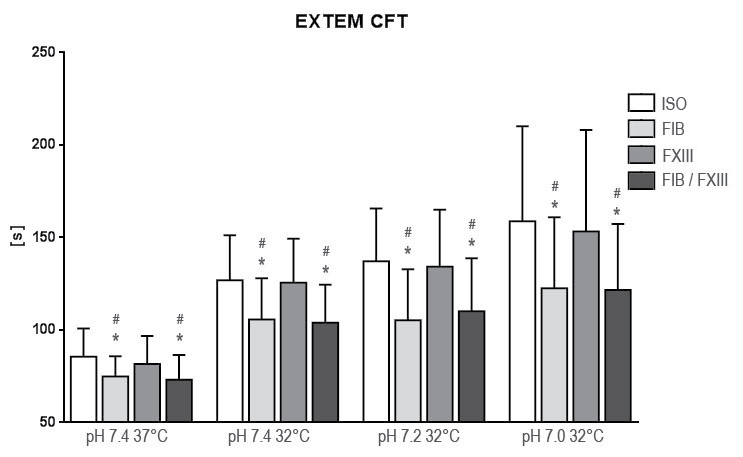
.
Figure 2: Results of rotation thrombelastometry clot formation time (CFT) after extrinsic activation under hypothermia and acidosis. Groups are Native: control group with addition of isotonic saline solution for compensation of dilutional effects; Fib: addition of Fibrinogen; FXIII: addition of Factor XIII; Fib FXIII: addition of both substances. Asterisks (*) mark significant differences to ISO, # mark significant differences to FXIII.
View Figure 2
ISO
At 32° Celsius and pH 7.4 CT and A20 were not significantly affected (CT: p = 0.553; A20; p = 0.202) while CFT was prolonged (p = 0.036). After simulating acidosis at 32° Celsius and pH 7.2 CT kept insignificant (p = 0.393) while CFT was prolonged (p = 0.006) and A20 (p = 0.016) was reduced significantly. These trends extended at 32° Celsius and pH 7.0: CT (p = 0.574), CFT (p < 0.001), A20 (A20; p < 0.001). By excluding platelet function we found fibrin polymerization not affected at all measuring points (pH 7.4 p = 0.631; pH 7.2 p = 0.600; pH 7.0 p = 0.736).
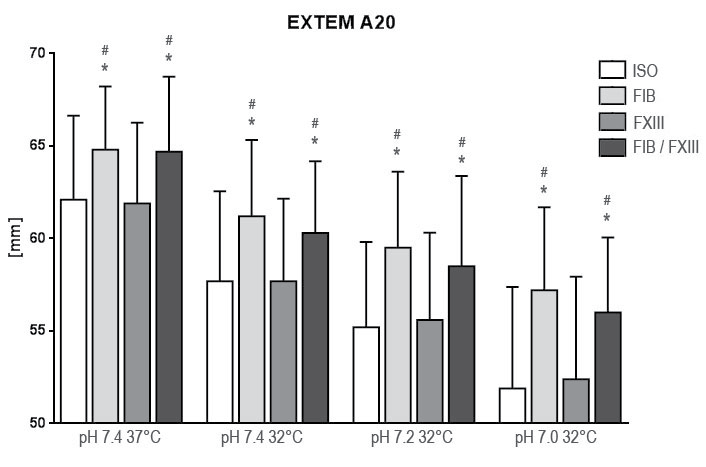
.
Figure 3: Results of rotation thrombelastometry maximum clot firmness (MCF) after extrinsic activation under hypothermia and acidosis. Groups are Native: control group with addition of isotonic saline solution for compensation of dilutional effects; Fib: addition of Fibrinogen; FXIII: addition of Factor XIII; Fib FXIII: addition of both substances. Asterisks (*) mark significant differences to ISO, # mark significant differences to FXIII.
View Figure 3
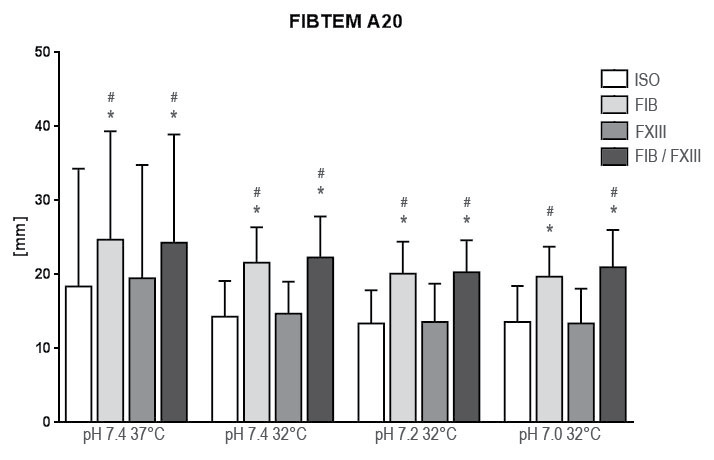
.
Figure 4: Results of fibrin polymerization after extrinsic activation under hypothermia and acidosis. Groups are Native: control group with addition of isotonic saline solution for compensation of dilutional effects; Fib: addition of Fibrinogen; FXIII: addition of Factor XIII; Fib FXIII: addition of both substances. Asterisks (*) mark significant differences to ISO, # mark significant differences to FXIII.
View Figure 4
FXIII+
As compared to ISO values FXIII+ did not affect any of the examined parameters significantly (CT, CFT, MCF, FIBTEM) at any measuring point.
FIB+
FIB+ on contrary significantly affected CT, CFT and MCF at all examination points as compared to ISO. Reconstitution of baseline values of CFT and MCF under hypothermia in EXTEM was not achieved at any pH-level. In FIBTEM however reconstitution of A20 in comparison to baseline was achieved at all pH-levels (p < 0.001).
FIB+FXIII+
FIB+FXIII+ affected all tested variables comparable to FIB+. Statistical significant differences to ISO were found for EXTEM A20 where FIB+ had a higher improvement than FIB+FXIII+ at all pH-levels at 32° Celsius (p = 0.029; 0.023; 0.044). CT, CFT, and FIBTEM A20 were insignificantly altered (CT: p = 0.331; CFT: p = 0.306; FIBTEM: p = 0.423).
Discussion
Coagulopathy is one main cause of mortality during major trauma in the early onset (46.0% with vs. 10.9% without coagulopathy) [1,4]. Two major aspects are responsible for development of coagulopathy, hypothermia and acidosis [15]. Treatment aims to restore basic conditions for blood coagulation such as achieving and maintaining normothermia, normal pH, and normal values for calcium, platelets and coagulation factors which is recommended by the guidelines for treatment of patients with major trauma [16]. While acidosis itself can be treated relatively easy and fast by application of sodium bicarbonate or tris buffer, hypothermia is a more severe fact to deal with in face of pressure of time. Kornberger et al. measured a mean rewarming rate of 1.7 degrees Celsius per hour examining fifteen hypothermic patients with a body core temperature varying from 24 to 30°C [17]. Although there are several options for rewarming hypothermic patients, it takes precious time. Ways of bridging this period of time and preventing coagulopathy are main subjects of interest. We could, referring to results we found in a preceding study, affirm that administration of fibrinogen improved all measured parameters: MCF (maximum clot firmness) and alpha-angle (gradient angle of a ROTEM tracing) increased while CT and CFT shortened. Furthermore, we reconfirmed that hypothermia and acidosis synergistically lead to decreased clot firmness whereas both, clotting time and clot formation time, are prolonged while fibrin polymerization was not affected by either hypothermia or acidosis [10].
Fibrinogen is beside platelet function and FXIII the most important substrate for clot firmness [13]. It takes part in primary as well as in secondary haemostasis [18]. By binding platelets via the glycoprotein IIb/IIIa receptor on the platelet's surface [19] it is involved in the early phase of aggregation. After fibrinogen activation by thrombin, fibrin builds a network that in turn incorporates platelets and proteins in the late onset of coagulation [20]. While the first compound of fibrin monomers is pretty loose it is cross-linked to covalent bounds by activated FXIII [9,21].
FXIII is the zymogen of a transglutaminase that has many different functions of which not all are well understood yet. It exists in two different forms, a tissue form and one that is located in the plasma. The tissue form is presented on platelets, macrophages and the placenta and plays an important role in maintaining pregnancy and promotes wound healing [9]. Patients suffering from FXIII-deficiency show wound healing problems as well as prolonged and severe bleeding [22,23]. In a prospective study on patients after intracranial surgery those with a decreased FXIII activity had a higher risk for postoperative hematoma [24]. The plasma form is activated by thrombin which results in a proteolytic removal of an amino-terminal propeptide and a following, calcium dependent conformation change [25]. Due to this change FXIIIa (activated FXIII) is able to build covalent cross-links of fibrin monomers to anneal clot firmness [9,25,26]. Furthermore, by attaching alpha-2-antiplasmin into the clot, FXIIIa also improves fibrinolysis-resistance and by engrafting fibronectin, collagen and other extracellular matrix proteins adhesion to the endothelium [8,9,25-28]. By inhibition of FXIII with antibodies in an In vitro study, Jambor et al. verified a reduction of maximum clot firmness while clot formation time and clot lysis have been increased [29]. Beside clot stabilization by cross-linking fibrinogen, glycoprotein IIb/IIIa, and cytoskeleton factors like actin and myosin [22,30], the underlying mechanisms are stimulating effects on angiogenesis and modeling of a matrix that ensures clot-adhesion as well as facilitating migration of cells that take part in tissue repair such as monocytes and fibroblasts [31,32].
Main cause for our findings may be the concentration-depended platelet link-rate of fibrinogen which can be increased by raising the fibrinogen concentration [33], even in presence of anti-platelet drugs as for example clopidogrel [34] as well as in patients suffering from thrombocytopenia [13,35]. There is evidence that high levels of fibrinogen may increase the risk for thrombosis, ischemic heart attack or stroke [36,37]. Dependency of clot stability from fibrinogen levels in a dose dependent manner [38] as well as a strong correlation of postoperative thromboembolic events and elevated values of clot firmness have been described [39]. In our study addition of FXIII showed no further benefit on clot formation under the given circumstances of hypothermia and acidosis - neither as single administration nor in combination with fibrinogen in comparison to the effect fibrinogen had exclusively. While our study participants showed normal FXIII values it could be possible that a ceiling effect of FXIII action can be responsible for our findings. In intensive care patients without hypothermia and acidosis but reduced FXIII levels a beneficial effect of FXIII on clot firmness has been described [40]. Thus, we only can speculate if addition of FXIII in presence of reduced FXIII levels may increase clot stability under hypothermia or acidosis. Furthermore, speed of FXIII activation could possibly be limited due to unknown cofactors, since FXIII, its functions and influences are not fully explored yet. Further research is necessary.
Limitations
In this study all volunteers were healthy and had normal FXIII levels. There might be some point of saturation from which there is no benefit by adding extra FXIII. It requires further studies to detect a possible benefit of FXIII to trauma patients with a possible acquired FXIII-deficiency. However, even if the implemented tests connect close to physiological processes, an In vitro examination cannot consider all of the complex incidents taking place in the critically ill patient. Furthermore, the artificially simulated acidosis can just rudimentary be compared with the acidosis developed during trauma with its origin in inadequate oxygen supply and shock. Nevertheless the used In vitro model of acidosis and hypothermia represents a standard model.
Acknowledgements
The authors would like to thank Brigitte Buchalik for excellent support in the laboratory.
Conflicts of Interests
AAH, MW, CFW, and NRM held scientific lectures on a fee basis and received funding for scientific work from CSL Behring and TEM International GmbH.
Funding
The presented study was performed without third party funding.
References
-
Brohi K, Singh J, Heron M, Coats T (2003) Acute traumatic coagulopathy. J Trauma 54: 1127-1130.
-
Pfeifer R, Tarkin IS, Rocos B, Pape HC (2009) Patterns of mortality and causes of death in polytrauma patients-has anything changed? Injury 40: 907-911.
-
Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, et al. (1995) Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185-193.
-
Misgav M, Martinowitz U (2011) [Trauma-induced coagulopathy--mechanisms and state of the art treatment]. Harefuah 150: 99-103, 207.
-
Dirkmann D, Hanke AA, Gorlinger K, Peters J (2008) Hypothermia and acidosis synergistically impair coagulation in human whole blood. Anesth Analg 106: 1627-1632.
-
Armand R, Hess JR (2003) Treating coagulopathy in trauma patients. Transfus Med Rev 17: 223-231.
-
Chambers LA, Chow SJ, Shaffer LE (2011) Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol 136: 364-370.
-
Hsieh L, Nugent D (2008) Factor XIII deficiency. Haemophilia 14: 1190-1200.
-
Muszbek L, Adany R, Mikkola H (1996) Novel aspects of blood coagulation factor XIII. I. Structure, distribution, activation, and function. Crit Rev Clin Lab Sci 33: 357-421.
-
Hanke AA, Dellweg C, Schochl H, Weber CF, Juttner B, et al. (2011) Potential of whole blood coagulation reconstitution by desmopressin and fibrinogen under conditions of hypothermia and acidosis--an In vitro study using rotation thrombelastometry. Scand J Clin Lab Invest 71: 292-298.
-
Fries D, Innerhofer P, Reif C, Streif W, Klingler A, et al. (2006) The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an In vitro model. Anesth Analg 102: 347-351.
-
Fries D, Haas T, Klingler A, Streif W, Klima G, et al. (2006) Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy--a porcine model. Br J Anaesth 97: 460-467.
-
Lang T, von Depka M (2006) [Possibilities and limitations of thrombelastometry/-graphy]. Hamostaseologie 26: S20-29.
-
Schochl H, Solomon C, Traintinger S, Nienaber U, Tacacs-Tolnai A, et al. (2011) Thromboelastometric (ROTEM) findings in patients suffering from isolated severe traumatic brain injury. J Neurotrauma 28: 2033-2041.
-
Mikhail J (1999) The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues 10: 85-94.
-
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, et al. (2010) Management of bleeding following major trauma: an updated European guideline. Crit Care 14: R52.
-
Kornberger E, Schwarz B, Lindner KH, Mair P (1999) Forced air surface rewarming in patients with severe accidental hypothermia. Resuscitation 41: 105-111.
-
Sørensen B, Tang M, Larsen OH, Laursen PN, Fenger-Eriksen C, et al. (2011) The role of fibrinogen: a new paradigm in the treatment of coagulopathic bleeding. Thromb Res 128 Suppl 1: S13-16.
-
Endenburg SC, Hantgan RR, Sixma JJ, De Groot PG, Zwaginga, JJ (1993) Platelet adhesion to fibrin(ogen). Blood coagulation & fibrinolysis? An International journal in haemostasis and thrombosis 4: 139-142.
-
Husain SS, Weisel JW, Budzynski AZ (1989) Interaction of fibrinogen and its derivatives with fibrin. J Biol Chem 264: 11414-11420.
-
Mosesson MW (1990) Fibrin polymerization and its regulatory role in hemostasis. J Lab Clin Med 116: 8-17.
-
Muszbek L, Yee VC, Hevessy Z (1999) Blood coagulation factor XIII: structure and function. Thromb Res 94: 271-305.
-
Muszbek L1 Bagoly Z, Cairo A, Peyvandi F (2011) Novel aspects of factor XIII deficiency. Curr Opin Hematol 18: 366-372.
-
Gerlach R, Tolle F, Raabe A, Zimmermann M, Siegemund A, et al. (2002) Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 33: 1618-1623.
-
Bagoly Z, Koncz Z, Harsfalvi J, Muszbek L (2012) Factor XIII, clot structure, thrombosis. Thromb Res 129: 382-387.
-
Romanic AM, Arleth AJ, Willette RN, Ohlstein EH (1998) Factor XIIIa cross-links lipoprotein(a) with fibrinogen and is present in human atherosclerotic lesions. Circ Res 83: 264-269.
-
Nourbakhsh E, Anvari R, D'cunha N, Thaxton L, Malik A, et al. (2011) Postoperative bleeding in a patient with normal screening coagulation tests. Am J Med Sci 342: 262-264.
-
Cox AD, Devine DV (1994) Factor XIIIa binding to activated platelets is mediated through activation of glycoprotein IIb-IIIa. Blood 83: 1006-1016.
-
Jambor C, Reul V, Schnider TW, Degiacomi P, Metzner H, et al. (2009) In vitro inhibition of factor XIII retards clot formation, reduces clot firmness, and increases fibrinolytic effects in whole blood. Anesth Analg 109: 1023-1028.
-
Wilmer M, Schroder V, Kohler HP (2002) Methods for the determination of factor XIII/XIIIa. Hamostaseologie 22: 32-42.
-
Dardik R, Loscalzo J, Inbal A (2006) Factor XIII (FXIII) and angiogenesis. J Thromb Haemost 4: 19-25.
-
Muszbek L, Bagoly Z, Bereczky Z, Katona E (2008) The involvement of blood coagulation factor XIII in fibrinolysis and thrombosis. Cardiovasc Hematol Agents Med Chem 6: 190-205.
-
Kanaide H, Shainoff JR (1975) Cross-linking of fibrinogen and fibrin by fibrin-stablizing factor (factor XIIIa). J Lab Clin Med 85: 574-597.
-
Li YF, Spencer FA, Becker RC (2001) Comparative efficacy of fibrinogen and platelet supplementation on the In vitro reversibility of competitive glycoprotein IIb/IIIa (alphaIIb/beta3) receptor-directed platelet inhibition. Am Heart J 142: 204-210.
-
Velik-Salchner C, Haas T, Innerhofer P, Streif W, Nussbaumer W, et al. (2007) The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost 5: 1019-1025.
-
Fibrinogen Studies Collaboration, Danesh J, Lewington S, Thompson SG, Lowe GD, et al. (2005) Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 294: 1799-1809.
-
Lowe GD, Lee AJ, Rumley A, Price JF, Fowkes FG (1997) Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol 96: 168-173.
-
Lang T, Johanning K, Metzler H, Piepenbrock S, Solomon C, et al. (2009) The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg 108: 751-758.
-
McCrath DJ, Cerboni E, Frumento RJ, Hirsh AL, Bennett-Guerrero E (2005) Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg 100: 1576-1583.
-
Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR (2010) In vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM). Thromb Haemost 104: 385-391.





