International Journal of Allergy Medications
Immunological Changes in Specific Oral Tolerance Induction for Cow's Milk Allergy
Carlos Alberto Sánchez Salguero1* and Álvaro Isidro Sánchez Chacón2
1Head of Pediatrics Allergy and Pneumology Section, School of Medicine, University Hospital Puerto Real, Cádiz, Spain
2Pediatrics Department, University Hospital Puerto Real, Cádiz, Spain
*Corresponding author:
Carlos Sanchez Salguero, Head of Pediatric Allergy and Pneumology Section, Professor of Pediatric, School of Medicine, University Hospital Puerto Real, Cadiz, Spain, E-mail: libraygeminis@hotmail.com
Int J Aller Medcations, IJAM-2-018, (Volume 2, Issue 2), Mini Review
Received: March 29, 2016 | Accepted: May 25, 2016 | Published: July 01, 2016
Citation: Salguero CAS and Chacon AIS (2016) Immunological Changes in Specific Oral Tolerance Induction for Cow's Milk Allergy. Int J Aller Medcations 2:018.
Copyright: © 2016 Salguero CAS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:The aim of this study was to evaluate the safety and efficacy of oral rush desensitization in children with cow milk allergy.
Material and methods:Prospective study: We included IgE-mediated cow milk allergy children over 3 years in 3 Spanish hospitals. Increasing doses of cow milk for 5 days from 1cc of 1% to 200 cc of pure milk were administered. Clinical follow-up was conducted and we compared specific IgE levels at onset, 6, 12 and 24 months after desensitization.
Results:We included 18 children (13 males) between 3 and 14 years (mean 5.96). A total of 271 doses were administered; there were 55 adverse reactions (84% mild). At the end of the protocol, 100% showed some degree of tolerance (39% total). Full tolerance was achieved in 72% of patients after two years. Two children failed to achieve tolerance. There was a significant decrease in the levels of specific IgE to cow milk and alpha-lactoalbumin at 24 months, and to casein at 6, 12 and 24 months, compared to baseline.
Conclusions:Oral rush desensitization is a safe and effective therapeutic option for patients with persistent cow milk allergy to medium term.
Keywords
Cow milk allergy, Oral tolerance induction, Oral rush desensitization
Introduction
The cow's milk proteins are the first food allergen to which the child is exposed, so they tend to be responsible for the first allergic reactions to foods that are manifested in atopic infants. The data provided by the international literature on the incidence of allergy to cow's milk are highly variable, because of conceptual differences, or diagnostic methodology ages studied, ranging from 0.3 to 7.5% [1-4]. In the study conducted in Denmark by Host, an incidence of 1.2% was observed in the first year of life [5]. Nationally, studies indicate that the incidence in the first year of life varies between 0.36 and 1.9% [6,7]. These data indicate that allergy to cow's milk protein, as the second most common food allergy in children, behind allergy to protein egg.
The natural history of allergies in childhood is favourable. The evolution points to the spontaneous appearance of tolerance in the medium to long term with disappearance of clinical [8]. In the natural history of food allergy, the clinical sensitization period followed by another awareness asymptomatic, up to the total tolerance, usually it accompanied by the disappearance of specific IgE antibodies. This good evolution occurs in patients with allergy to cow's milk protein up to 83% at 5 years of life [9]. If tolerance is not reached at the usual time it is considered persistent allergy and can be accompanied by severe clinical, even minimal amounts intake of food [10].
Specific Oral Tolerance Induction to food (SOTI) consists of the oral administration of food allergen causing symptoms, in this case the proteins of cow's milk, starting with minimum and gradually increasing amounts until reaching standard ration for age or maximum tolerated dose threshold [11-13]. This is to establish an immunological tolerance, re-educating the complex cellular and serological mechanism to correct an inadequate reaction through a process of gradual increase in the threshold amount tolerated.
This treatment option has been used mainly in patients with allergies to milk proteins persistent cow, which due to the natural evolution of this allergy, arrived at a certain time was unlikely to reach passive tolerance. However, in recent years, it is trying to induction of oral tolerance at younger ages in order to obtain better results and minimize risks [14].
There is no single pattern of standardized SOTI. Each centre, according to its available means and patient characteristics, uses a pattern based on proposed by scientific societies or used by other professionals. In general, the different protocols could be classified: by duration (rush or quick, slow, intermediate or mixed), depending on where it takes place (entered with partial income, with increases in hospital, increases in home, weekly, daily ... increments) and according to receive or not premedication for its realization.
Currently, there are randomized trials demonstrating short-term efficacy of such protocols, with a success rate of up to 90% [15,16]; however, there are some doubts about their safety. Since this treatment option has been applied for the last decade, there are no studies that allow us to know the long-term effects in these patients [17].
The aim of our study was to evaluate the immunological changes induced by SOTI, and in second place evaluated the safety of a rush protocol.
Materials and Methods
Multicentre prospective longitudinal study involving three hospitals in Cadiz province (Spain) (University Hospital Puerto Real, University Hospital Puerta del Mar and University Hospital Jerez). Inclusion criteria: children over three years with an allergy to cow's milk protein IgE-mediated defined by presenting specific IgE positive (> 0.35 kU/L) to cow's milk, casein, α-lactoalbumin and β-lactoglobulin and controlled exposure test to previous positive antigen desensitization, except in patients with a history of recent anaphylactic reaction. Each hospital contributed with 6 patients, the first 6 patients than needed desensitization to cow's milk allergy.
The induction of oral tolerance was performed in 5 days with the inpatient hospital day. Increasing doses were administered milk, from a starting dose of 1cc 1% (99 cc of water and milk 1cc) to 200 ml of whole milk. The dosing schedule is shown in table 1. No medication was used prior to administration. All adverse reactions taking place during the administration of different doses were recorded. To quantify the severity classification Clark (Table 2) [13] was used. If by administering a dose patients had mild adverse reactions (rash, urticarial, oral allergy, rhinitis ...) is continued increases, whereas if the reactions were severe (angioedema, severe bronchospasm, shock ...), the test was discontinued and it restarted the next day with the next lowest dose at which triggered the event (Table 2).
![]()
Table 1: Protocol oral tolerance induction by pattern rush.
View Table 1
![]()
Table 2: Clark classification of allergic adverse reactions.
View Table 2
It is considered that patients develop total tolerance when they were able to tolerate at least 6.600 mg protein of cow's milk per day (equivalent to that contained in 200 cc of whole milk) and partial tolerance when they were able to tolerate lower amounts without clinically effects.
Specific IgE levels were quantified: whole milk, casein, α-lactoalbumin and β-lactoglobulin. Baseline measurement prior to beginning the protocol and then at 6, 12 and 24 months after completion of it was made. The collected data were analysed using the Statistical Package for the Social Sciences (SPSS) version 15.0. Basic descriptive techniques were applied, expressing the results as median and range in parentheses. For comparison of specific IgE levels prior to desensitization at 6, 12 and 24 months Wilcoxon test was applied. Significant at p < 0.05 were considered.
Results
There were included 18 patients (13 males), aged between 3 and 14 years (mean ± standard deviation 5.96 ± 0.75 years). 82% had a clinical history of asthma, 27% concomitant sensitization to aeroallergens and 33% to other aero-allergens. Two patients had a history of anaphylactic reaction secondary to accidental intake of milk.
Specific IgE levels, expressed as median and range in brackets before performing desensitization were: α-lactoalbumin 2.28 (0.4 to 42) kU/L, β-lactoglobulin 0.85 (0.12-15) kU/L, casein 6.33 (0.34 to 100) kU/L cow's milk 5.80 (0.82 to 168) kU/L. 83% of patients had some type of adverse reaction after receiving the appropriate dose, all within 30 min after ingestion. Adverse reactions were observed in 20.3% of 271 doses administered (55 in total). The vast majority of the reactions observed were mild (84% type 1-3 Clark classification). In one dose an anaphylactic reaction occurred. 47 times antihistamines, corticosteroids in 7, nebulized salbutamol in 12 and intramuscular adrenaline were administered once.
At the end of the protocol, all patients achieved some degree of tolerance (39% overall, 61% partial). The average amount was 103 ± tolerated 77 (5-200) ml of whole milk. Patients with partial tolerance received daily at home the last dose were able to tolerate the fifth day of the SOTI and weekly increments were performed in the hospital following the same protocol performance (Table 1). After 2 years of follow-up, tolerance was 149 ± 71 (0-200) ml of whole milk and 72% of patients performed a restriction diet without dairy products.
Two patients lost their tolerance they had acquired. The first of them, 2 months after the finishing of the protocol, he began with oral allergy symptoms and vomiting and abdominal pain (Clark 1-3), which gradually decreased milk intake until complete suspension at 12 months. Their average levels of specific IgE to cow's milk and casein decreased significantly at 6 and 12 months later rise to 2 years SOTI. The second, after finishing the protocol, did not continue at home with the recommended daily dose of milk, when he tried to reintroduce it in the hospital suffered an anaphylactic reaction and did not try desensitization.
Specific IgE levels to cow's milk and α-lactoalbumin at 24 months showed a significant decrease from baseline of desensitization: 2.83 (1.26 to 18) kU/L (p = 0.043) and 1.79 (0.62 to 4.58) kU/L (p = 0.043), respectively. Casein levels were significantly lower at 6, 12 and 24 month desensitization: 4.69 (0.68 to 24.30) kU/L (p = 0.028), 2.42 (0.35 to 8, 02) kU/L (p = 0.012) and 0.73 (0.14 to 17.30) kU/L (p = 0.043). No differences in the levels of β-lactoglobulin found. Serum levels of specific IgE to cow's milk proteins shown in figure 1.
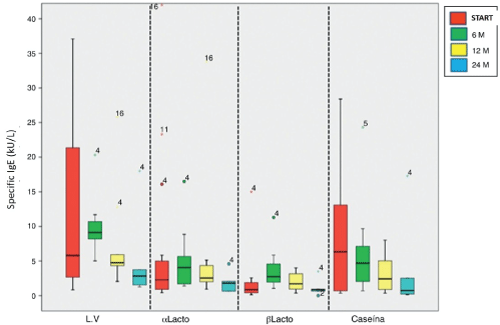
.
Figure 1: Serum levels of IgE specific to cow's milk proteins at baseline and at 6, 12 and 24 months after desensitization. The upper and lower edges of the boxes correspond to the 75th and 25th percentiles, the centre line that divides indicate the median and whiskers, the upper and lower limits.
LV: cow's milk; m: months; αlacto: α-lactoalbumin; βlacto: β-lactoglobulin.
View Figure 1
Discussion
The Specific Oral Tolerance Induction to food began to perform in a majority in the last decade. The results of the studies published to date are quite encouraging, with a success rate in the short term between 40 and 90% [15,16,18], figures similar to our study, where all patients achieved some degree of tolerance after completion of induction protocol and up to 89% after 2 years of follow-up. However, there are still many unresolved issues, primarily related to security and complications medium and long term of this new therapeutic option.
The appearance of symptoms of varying severity during the procedure and the need for repeat doses is practically constant in all protocols used for induction of oral tolerance. Most of these symptoms are not severe and usually require no medication or enough with the administration of oral antihistamines. This pattern is repeated in our series, where more than half of our patients suffered some sort of reaction, with most of them mild.
Moreover, in the series published to date, no small percentage of patients (5-15%) had severe reactions and required the administration of epinephrine intramuscularly both in hospitals and in their homes [16,18,19]. Barbi et al. [20,21] using a hospital initial rush pattern increments at home in 132 patients with milk protein allergy severe cow (specific IgE > 100 kU/L). In this series, 64% of patients had some kind of reaction at home, being 27% of them secondary to increased formula, requiring the administration of adrenaline 4% of patients.
In our study, only one patient required adrenaline and just reactions occurred at home. The rush pattern made entirely in the hospital decreases the likelihood of reactions at home, reduced to those cases in which a failure of tolerance induction or be triggered by strenuous exercise or infection occurs because the increases provided they are performed in a hospital environment.
Further studies are needed to know whether this risk is similar to having a severe reaction after accidental ingestion in patients not receiving treatment by desensitization [22-24]. Therefore, it is very important to strengthen surveillance measures in these patients, educate the family and themselves on recognizing and treating the symptoms, not physical exercise within 3 hours after the daily intake of milk; as decrease the amount coinciding with infectious processes and increase it once surpassed these [25].
It must have a call service to assist patients and use desensitization protocols in which at least the initial phases and increments are carried out in a hospital setting and in units with trained personnel and sufficient means of controlling severe reactions that may arise during the process.
Immunologic mechanisms that explain the phenomenon of induction of oral tolerance are still unknown. The values of specific IgE, along with age and personal history of severe asthma or anaphylactic reaction, are circumstances that we must consider when ask oral treatment by desensitization. The 3 patients with levels above 30 kU/L at baseline IgE specific protocol were those who raised us greater difficulties; 2 of them despite completing the protocol, lost progressively acquired tolerance for different reasons and another had an anaphylactic reaction during induction, finally reaching a tolerance of 50 cc of whole milk after 2 years of follow-up.
Specific IgE values tend to lower very slowly, remaining similar or higher to achieve maximum tolerated and then decrease over the next 12-18 months [26,27]. In our study, with follow-up of 2 years, we observed a decrease at 12 and 24 months of all levels of IgE specific, except those of β-lactoglobulin. If we analyse the levels of IgE in the patient with loss of tolerance, we observed during the first 12 months, while continuing treatment, levels gradually decreased; however, the patient had frequent reactions despite lowering the daily dose of milk. After stopping treatment, levels of α-lactoalbumin and casein rose again.
Therefore, IgE levels were not useful to distinguish which patient failed induction of oral tolerance. The fact that the immunological changes were observed post suggests that specific IgE play a secondary role with respect to tolerance, being an epiphenomenon of other immunological changes in relation to regulatory T cells and cytokines, whose knowledge and modulation are the current focus of research in this field.
Despite high success rate, defined as partial or complete tolerance after completing the desensitization protocol in the literature, any case of loss of tolerance is quoted after the suspension, for a few months, food and tolerated by SOTI. This phenomenon can occur in both, natural tolerance as in induced tolerance [28,29]. Meglio et al. [27], after a follow up of 4 years, watched all patients who had completed the desensitization protocol maintained their level of tolerance except 3 patients, 2 of them maternally decided to suspend the treatment decision and one that after an episode of viral gastroenteritis, she was a month without eating milk and when he tried to reintroduce it failed due to the occurrence of urticarial type reactions and asthma.
This circumstance is again to show in our series, where a patient mistakenly stopped using their daily dose of milk that had reached the end tolerate the protocol and when we tried to re-enter the patient had an anaphylactic reaction. Some authors [25] include in its protocols the systematic suspension of food after reaching tolerance and are up to 25% relapse. All these findings suggest that tolerance may not be permanent.
Is unknown how long it should continue taking food for tolerance is maintained, an accepted recommendation is the following: during the induction stage and throughout the first 12 months you should ensure daily intake of a normal diet (200 ml milk) to ensure tolerance and then the food should be incorporated into the diet, without force and without daily control of quantities, like any other child. These recommendations are based in the protocol made by the Spanish Society of Pediatrics Clinical Immunology, Allergy and Asthma.
In recent years, there have been confirmed and suspected cases 20 primary eosinophilic disorders [30,31], mainly as eosinophilic esophagitis as a cause of failure of oral tolerance induction foods. Studies to confirm what the real association between the two processes are needed. Mixed allergic mechanisms are responsible for the appearance of primary eosinophilic disorders; therefore, not mediated allergy IgE should have a role in this association. Moreover, the SOTI appears to modify the natural history of food allergy, causing a gradual increase in the threshold amount tolerated for the development of certain adverse reactions; however, continuous exposure to the allergen may induce gastrointestinal level development of such disorders, like EoE [32,33].
We are aware of the limitations of our study mainly marked by the absence of placebo control group or other patterns of oral tolerance induction does not allow us to compare the effectiveness of the pattern rush with other therapeutic options.
Our data show that the induction of oral tolerance to cow's milk proteins by a rush regimen, in patients with moderate levels of specific IgE, is safe and effective after 2 years of follow-up. The rush tones main advantages speed and lower probability of reactions at home. It is an outpatient procedure that cannot be accomplished by the risks they entail. IgE levels down after 2 years of follow-up; however, they do not seem to predict the clinical course of patients.
Conflict of Interests
The authors declare no conflict of interest.
References
-
Høst A (1994) Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr Allergy Immunol 5: 1-36.
-
Halken S (2004) Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 15: 9-32.
-
Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, et al. (2015) Incidence and natural history of challenge-proven cow's milk allergy in European children - EuroPrevall birth cohort. Allergy 70: 963-972.
-
Venter C, Arshad SH (2011) Epidemiology of food allergy. Pediatr Clin North Am 58: 327-349.
-
Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, et al. (2002) Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol 15: 23-28.
-
Sanz Ortega J,Martorell Aragones A, Michavila Gómez A, Nieto García A; Grupo de Trabajo para el Estudio de la Alergia Alimentaria (2001) Incidence of IgE-mediatedallergy to cow'smilkproteins in thefirstyear of life. An Esp Pediatr 54: 536-539.
-
Garcia Ara MC, Boyano Martinez MT, Diaz Pena JM, Martin Munoz F, Pascual Marcos C, et al. (2003) Incidence of allergy to cow's milk and its impact on consumption hydrolysates. An Pediatr 58: 100-105.
-
Savage J, Sicherer S, Wood R (2016) The Natural History of Food Allergy. J Allergy Clin Immunol Pract 4: 196-203.
-
Martorell A, Plaza AM, Bone J, Nevot S et al. (2006) Cow's milk protein allergy. A multicenter study: clinical and epidemiological aspects. Allergol Immunopathol 34: 46-53.
-
Alvarado MI, Alonso E, GarciaAlvarez M, Ibanez MD, Laso MT (2000) Persistence of sensitization to cow's milk proteins: clinical study. Allergol Immunopathol 28: 189.
-
Alonso-Lebrero E, Fuentes Aparicio V, Zapatero Remon L (2010) Induction of tolerance in food allergy. Bol Pediatr 50: 80-86.
-
García-Ara C, Pedrosa M, Belver MT, Martín-Munoz MF, Quirce S, et al. (2013) Efficacy and safety of oral desensitization in children with cow's milk allergy according to their serum specific IgE level. Ann Allergy Asthma Immunol 110: 290-294.
-
Longo G, Berti I, Barbi E, Calligaris L, Matarazzo L, et al. (2012) Diagnosed child, treated child: food challenge as the first step toward tolerance induction in cow's milk protein allergy. Eur Ann Allergy Clin Immunol 44: 54-60.
-
Martorell A, De la Hoz B, Ibánez MD, Bone J, Terrados MS, et al. (2011) Oral desensitization as a useful treatment in 2-year-old children with cow's milk allergy. Clin Exp Allergy 41: 1297-1304.
-
Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, et al. (2008) A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol 122: 1154-1160.
-
Pajno GB, Caminiti L, Ruggeri P, De Luca R, Vita D, et al. (2010) Oral immunotherapy for cow's milk allergy with a weekly up-dosing regimen: a randomized single-blind controlled study. Ann Allergy Asthma Immunol 105: 376-381.
-
Sopo SM, Onesimo R, Giorgio V, Fundarò C (2010) Specific oral tolerance induction (SOTI) in pediatric age: clinical research or just routine practice? Pediatr Allergy Immunol 21: e446-449.
-
Clark AT, Ewan PW (2003) Food allergy in childhood. Arch Dis Child 88: 79-81.
-
Caminiti L, Passalacqua G, Barberi S, Vita D, Barberio G, et al. (2009) A new protocol for specific oral tolerance induction in children with IgE-mediated cow's milk allergy. Allergy Asthma Proc 30: 443-448.
-
Barbi E, Longo G, Berti I, Matarazzo L, Rubert L, et al. (2012) Adverse effects during specific oral tolerance induction: in home phase. Allergol Immunopathol (Madr) 40: 41-50.
-
Barbi E, Longo G, Berti I, Neri E, Saccari A, et al. (2012) Adverse effects during specific oral tolerance induction: in-hospital "rush" phase. Eur Ann Allergy Clin Immunol 44: 18-25.
-
Martorell-Calatayud C, Michavila-Gomez A, Martorell-Aragones A, Molini-Menchon N, Cerda-Mir JC, et al. (2016) Anti-IgE-assisted desensitization to egg and cow's milk in patients refractory to conventional oral immunotherapy. Pediatr Allergy Immunol.
-
Meglio P, Caminiti L, Pajno GB, DelloIacono I, Tripodi S, et al. (2015) The oral food desensitization in the Italian allergy centres. Eur Ann Allergy Clin Immunol 47: 68-76.
-
González Jimenez D, Larrea Tamayo E, Díaz Martin JJ, Molinos Norniella C, Perez Solis D, et al. (2013) Oral rush desensitization for cow milk allergy: Clinical and immunological follow-up. An Pediatr (Barc) 79: 346-351.
-
Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, et al. (2007) Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 62: 1261-1269.
-
Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, et al. (2008) Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol 121: 343-347.
-
Meglio P, Giampietro PG, Gianni S, Galli E (2008) Oral desensitization in children with immunoglobulin E-mediated cow's milk allergy-follow-up at 4 yr and 8 months. Pediatr Allergy Immunol 19: 412-419.
-
Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, et al. (2005) Specific oral tolerance induction with food in children: Transient or persistent effect on food allergy? Allergy 60: 1320-1322.
-
Longo G, Barbi E, Berti I, et al. (2008) Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol 121: 343-347.
-
Ros Garcia M, Tovar Y, Sanchez Garcia S, Ibanez P, Munoz Codoceo R (2011) Induction of tolerance in food allergy (SOTI): eosinophilic esophagitis? Rev Esp Pediatr 67: 70.
-
Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT (2011) Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol 127: 1622-1624.
-
Ridolo E, De Angelis GL, Dall'aglio P (2011) Eosinophilic esophagitis after specific oral tolerance induction for egg protein. Ann Allergy Asthma Immunol 106: 73-74.
-
Fuentes-Aparicio V, Alvarez-Perea A, Infante S, Zapatero L, D'Oleo A, et al. (2013) Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergol Immunopathol 41: 143-150.





