Balanced anaesthesia relies on the administration of opioids in the perioperative period as antinociceptive agents. There is no clear evidence that intraoperative opioids result in reduction of postoperative pain scores. Opioid-free anaesthesia (OFA), combination of various opioids-sparing techniques leading to no administration of intraoperative systemic, neuraxial or intracavitary opioids, arises from the attempt to develop anti-hyperalgesic techniques to improve postoperative pain control. Therefore, the aim of this review is to understand to which extend is opioid free beneficial in the perioperative period, more specifically the analgesic impact of this technique.
The electronic databases Medline and PubMed were searched until November 2019. We included meta-analyses, randomized controlled trials and prospective studies investigating pain outcomes comparing any type of intra-operative opioid general anaesthesia with opioid-free general anaesthesia. The primary outcome was first measure of pain score at rest and at 24 postoperative hours. Secondary outcomes included rescue analgesia, intravenous (i.v.) morphine consumption equivalents at 24h postoperatively, rates of postoperative nausea and vomiting (PONV) within the first 24 postoperative hours, rates of rescue antiemetic drugs, length of stay in post-anaesthesia care unit (PACU) and total hospital length of stay. Eleven studies were identified, three of which are meta-analysis.
Mean pain scores at rest in the first measure as well as at 24 postoperative hours were lower in the opioid free anaesthesia (OFA) group than in opioid based anaesthesia (OBA). Use of rescue postoperative analgesia and i.v. morphine consumption equivalents were lower in the OFA group. A statistically significant trend toward a decrease in PONV and use of antiemetic drugs among patients who did not received opioids was observed. Length of stay PACU was longer in the opioid free group, but only three of six trials reported a statistically significant difference. Finally, total length of stay in the hospital was investigated by two trials and was similar between groups.
OFA, when compared with OBA, does not present inferior results regarding pain scores or opioid consumption in the postoperative period. It is also associated with reduced postoperative nausea and vomiting. The OFA technique presents as future challenges an objective documentation of both its short-term and long-term benefits and inconveniencies. Further research with robust methodological trials with large sample sizes are required to better determine the efficacy and safety of opioid-free anaesthetic strategy.
Opioid-free anaesthesia, General anaesthesia, Analgesia, Opioid, Perioperative period, Multimodal treatment
The definition of general anaesthesia is a fluid term, having had several definitions. General anaesthesia was initially considered to be the four "A's": Analgesia, amnesia, akinesia (immobility) and autonomic control [1]. This concept evolves into the definition of General anaesthesia as a reversible state of unconsciousness, immobility, antinociception and control of autonomic nervous system (ANS), within a controlled hemodynamic physiological stability [2]. Another essential outcome that can only be retrospectively assessed is amnesia, which is assumed when patients are unconscious. It is currently believed that when the other four outcomes mentioned above are achieved, awareness with recall rarely occurs [3,4].
Balanced anaesthesia, the most common strategy used in the last decades, relies mostly upon the GABA-A receptor and mu-opioid receptor [3]. So the current practice is based on a hypnotic for induction and on an inhaled ether or hypnotic for maintenance of unconsciousness. Muscle relaxants are administered to produce immobility and opioids are the most commonly used drug to manage nociception intraoperatively and pain postoperatively [2,3]. Opioids used to be the ideal drug to block autonomic nervous system reactions and allow hemodynamic stability [2,5].
Nociception is intimately related to control of the autonomic nervous system since nociceptive disorders are a primary source of hemodynamic instability, as well as, postoperative chronic pain syndromes [6].
A sufficient level of antinociception has been reached when applied surgical stimuli, clinical responses as heart rate and blood pressure elevations no longer occur [2,3,5]. Nevertheless, according to a recent clinical trial, despite absent clinical responses, nociceptive activation persists during deep general anaesthesia. Therefore, lack of clinical responses is not indicative of the absent nociception specific activation [7].
Undoubtedly, opioids are effective antinociceptive agents and one of the three pillars of balanced anaesthesia is the administration of opioids in the perioperative period [3,8].
The approach of opioid administration before surgery has been used as a strategy to reduce postoperative pain. However, a recent meta-analysis of 20 randomised controlled trials concluded that there is no clear evidence that preventive opioids result in reduction in pain scores [9]. It is also known that perioperative opioid administration predisposes to persistent opioid use [10].
A 2014 meta-analysis that evaluated the clinical consequences of intraoperative doses of opioid, revealed that high doses of opioids during surgery are associated with an increased perception of pain and increased postoperative opioids requirements [11].
This can be explained by two related phenomena: Opioid tolerance and opioid-induced hyperalgesia [12,13]. Tolerance is a pharmacological effect that leads to a progressive lack of response to opioid administration that can be overcome by increasing the dose. Opioid-induced hyperalgesia is a sensitization process whereby opioids, paradoxically, cause increased pain sensitivity (Opioid Paradox) [8,12]. These neuroadaptation processes cause a highlighting of existing pain and enablement of chronic pain development [12,13]. Any opioid is capable of potentially inducing hyperalgesia, particularly short-acting opioids [14].
Although opioids are the most effective antinociceptive drug, they have undesirable side effects, such as respiratory depression, pharyngeal muscle weakness, postoperative nausea and vomiting, urinary retention, constipation, ileum, pruritus, tolerance and hyperalgesia that may progress into chronic pain syndrome [8,13,15]. Nausea and vomiting are particularly responsible for delayed patients recovery, prolonged patient stay in the recovery area and, therefore, delayed hospital discharge. It is also known that opioids disorganize sleep pattern and may lead to postoperative delirium [16]. Also, patients receiving opioids as part of general anaesthesia and leaving the hospital with opioid prescriptions, appear to have an increased risk of opioid dependence [17]. Therefore, it is debatable whether perioperative opioid administration is appropriate or necessary in current clinical practice [18].
Due to concerns arising from the excessive use of opioids and their side effects, new strategies have emerged to achieve balanced general anaesthesia. The concept of balanced anaesthesia has been extended in order to include more drugs that target different neurophysiologic mechanisms [2,3]. It is known that when anaesthetic drugs with different mechanisms are combined, they produce a synergic interaction, meaning that using different drugs at smaller doses maximizes desired effects while minimizing side effects. This phenomenon is known as Multimodal general anaesthesia and has allowed the dose reduction of opioids used [3,19]. Moreover, a multimodal approach can potentially reduce central neuroadaptation to opioids [17].
Concerned about the significant opioid side effects, strategies for balanced general anaesthesia are now using different antinociceptive agents that target the central nervous system, like dexmedetomidine [20], and less specific targets, like lidocaine [21], to manage the nociceptive component of anaesthesia [2].
Nonopioid adjuvants such as NSAIDs, beta-blockers, NMDA antagonists (ketamine), alpha2-agonists, lidocaine, gabapentin, etc. can decrease the need for opioids to achieve adequate intraoperative antinociception or post-operative analgesia [8,13]. Nevertheless, we should keep in mind that they must be chosen based on the patient and procedure for which their pharmacologic profile suits best [22]. Most of these drugs are able to decrease intraoperative opioid use at the cost of longer sedation [8].
Due to the new reasons that are added every year for the reduction of opioid use associated with the broadening of the concept of general multimodal anaesthesia, a new concept has emerged: Opioid-free anaesthesia. Opioid-free anaesthesia (OFA) can be defined as the combination of various opioids-sparing techniques leading to no administration of intraoperative systemic, neuraxial or intracavitary opioids [8,14,15]. OFA can also be performed with locoregional analgesia for better pain control, but it is not compulsory [14]. This strategy helps to reduce the incidence of opioid-induced adverse effects and spares opioids as analgesics for the postoperative period [16].
There are specific populations that benefit from the use of OFA, namely in opioid addiction, chronic pain syndromes, obesity, obstructive sleep apnoea, cancer surgery and colorectal ERAS (Figure 1) [8,13-15].
 Figure 1: Specific populations that benefit from the use of OFA technique.
View Figure 1
Figure 1: Specific populations that benefit from the use of OFA technique.
View Figure 1
Even though perioperative opioid administration is a common and long-standing practice, it is questionable whether it is still necessary in current practice. Therefore, the aim of this review is to understand to which extend is opioid free beneficial in the perioperative period, more specifically the analgesic impact of opioid-free anaesthesia.
The search for this review was performed on PubMed and Medline until 30 November 2019 using the query: ("opioid-free"[All Fields] AND (((("anaesthesia"[All Fields] OR "anesthesia"[MeSH Terms]) OR "anesthesia"[All Fields]) OR "anaesthesias"[All Fields]) OR "anesthesias"[All Fields])) OR ((("opioid-free"[All Fields] OR "non-opioid"[All Fields]) OR ("intra-operative"[All Fields] AND (((((("analgesics opioid"[Pharmacological Action] OR "analgesics, opioid"[MeSH Terms]) OR ("analgesics"[All Fields] AND "opioid"[All Fields])) OR "opioid analgesics"[All Fields]) OR "opioid"[All Fields]) OR "opioids"[All Fields]) OR "opioid's"[All Fields]))) AND (((("anaesthesia"[All Fields] OR "anesthesia"[MeSH Terms]) OR "anesthesia"[All Fields]) OR "anaesthesias"[All Fields]) OR "anesthesias"[All Fields])). Finally, Google Scholar was also used to identify any relevant study not already identified using the strategy described above.
The results of this search strategy were limited to meta-analyses, randomized controlled trials, prospective studies and humans, written in English or Portuguese. The analysis focus only on articles published in the last 5 years (from 30.11.2014 to 30.11.2019). Articles in other languages, systematic or literature reviews, retrospective studies, case reports, personal opinion articles and letters to the editor were excluded.
Titles and abstracts were screened in the first stage based on inclusion and exclusion criteria. Only trials that included patients under general anaesthesia and investigated pain outcomes comparing any type of intra-operative opioid administration with absence of opioids were included in the present review. Eligibility criteria was only applied to primary studies.
The primary outcome to be evaluated will be first measure of pain score at rest and at 24 postoperative hours. The secondary outcome elated to acute pain included need for rescue analgesia and intravenous (i.v.) morphine consumption equivalents at 24h postoperatively. Other secondary outcomes sought were rates of postoperative nausea and vomiting (PONV) within the first 24 postoperative hours, rates of antiemetic drugs, and hospital resource-related outcomes including length of stay in post-anaesthesia care unit (PACU) and the total hospital length of stay.
Extracted trial characteristics included: Surgical procedure, intra-operative opioid regimen, medication used for anaesthetic maintenance and type of postoperative analgesia.
Briefly, the Cochrane Collaboration's Risk of Bias Tool for randomized controlled trials was used to assess the methodological quality of each randomised trial [23]. No attempt was made to contact authors for clarification on the Risk of Bias items. Selective outcome reporting was judged based on the outcomes described in the Methods section but not reported in the Results section.
If in any study the data was found to be incomplete, attempts were made to contact the corresponding author via email for the relevant data. In one study, pain scores were presented in a graphical format [24]. Therefore, an online software (https://apps.automeris.io/wpd/) was used to extract data points.
Outcomes are reported as tables and relevant outcomes for each study are summarized. All opioids were converted into equianalgesic doses of i.v. morphine for analysis (i.v. morphine 10 mg = oral morphine 30 mg = i.v. pethidine 75 mg = i.v. piritramide 7.5 mg) [25,26]. Pain scores reported as visual, verbal or numeric rating scales were converted to a standardised 0-10 analogue scale for quantitative evaluations. Comparative effectiveness is reported either as proportion of patients with the outcome or as mean score. The p value < 0.05 was considered significant.
Of the 1415 studies identified by our literature search, 11 met the inclusion criteria, representing a total of 3483 patients (2975 from meta-analysis and 508 from trials) [24,27-36]. The flowchart following PRISMA guidelines shows the search and selection process of the literature (Figure 2).
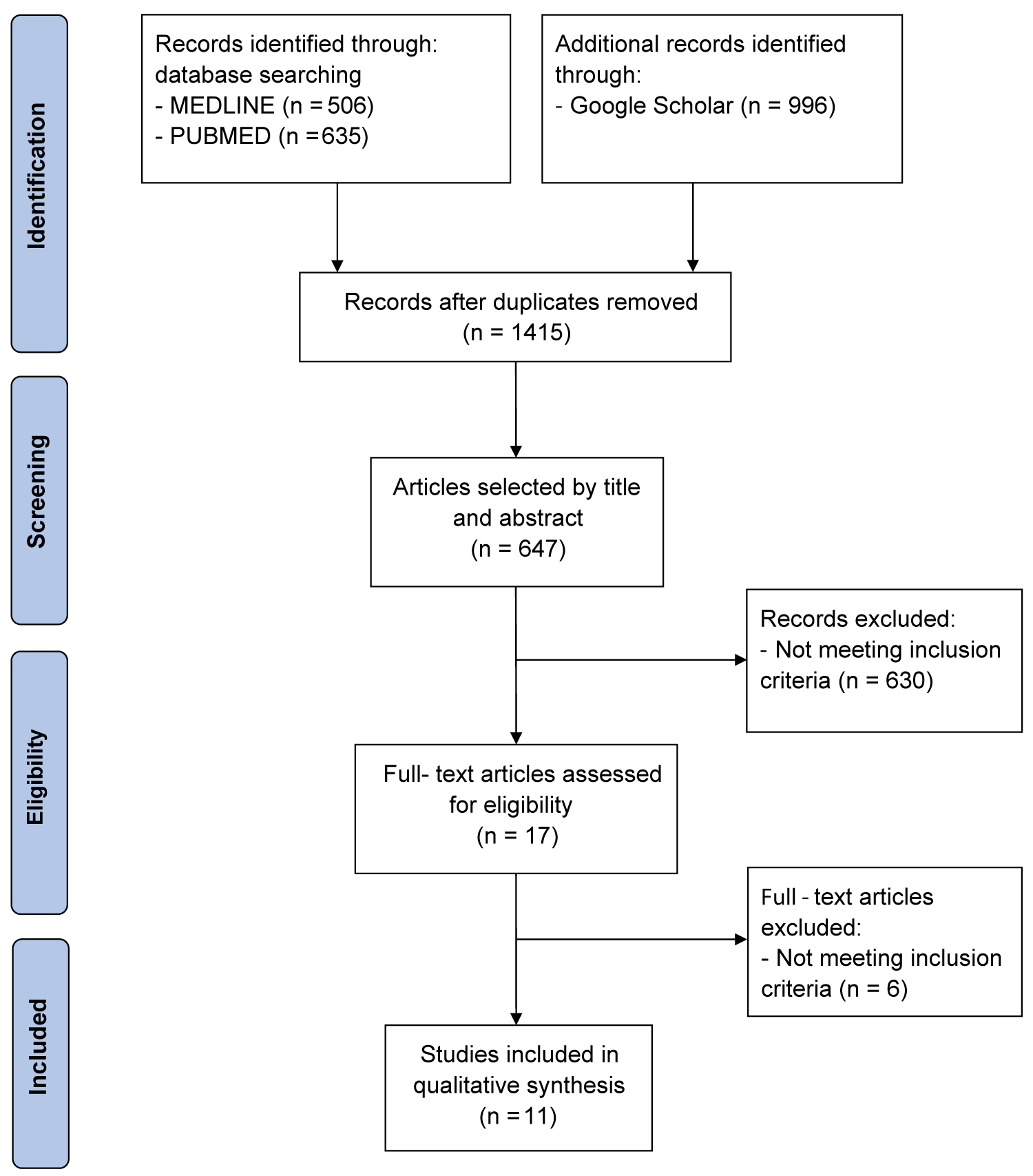 Figure 2: PRISMA flow diagram showing literature search results. Eleven articles were included in the analysis.
View Figure 2
Figure 2: PRISMA flow diagram showing literature search results. Eleven articles were included in the analysis.
View Figure 2
According to our assessment following the Cochrane Collaboration Risk of Bias tool (Figure 3), the majority of trials had an unclear risk of bias. Attempts were made to contact three authors [24,34,35], but none provided the additional data requested.
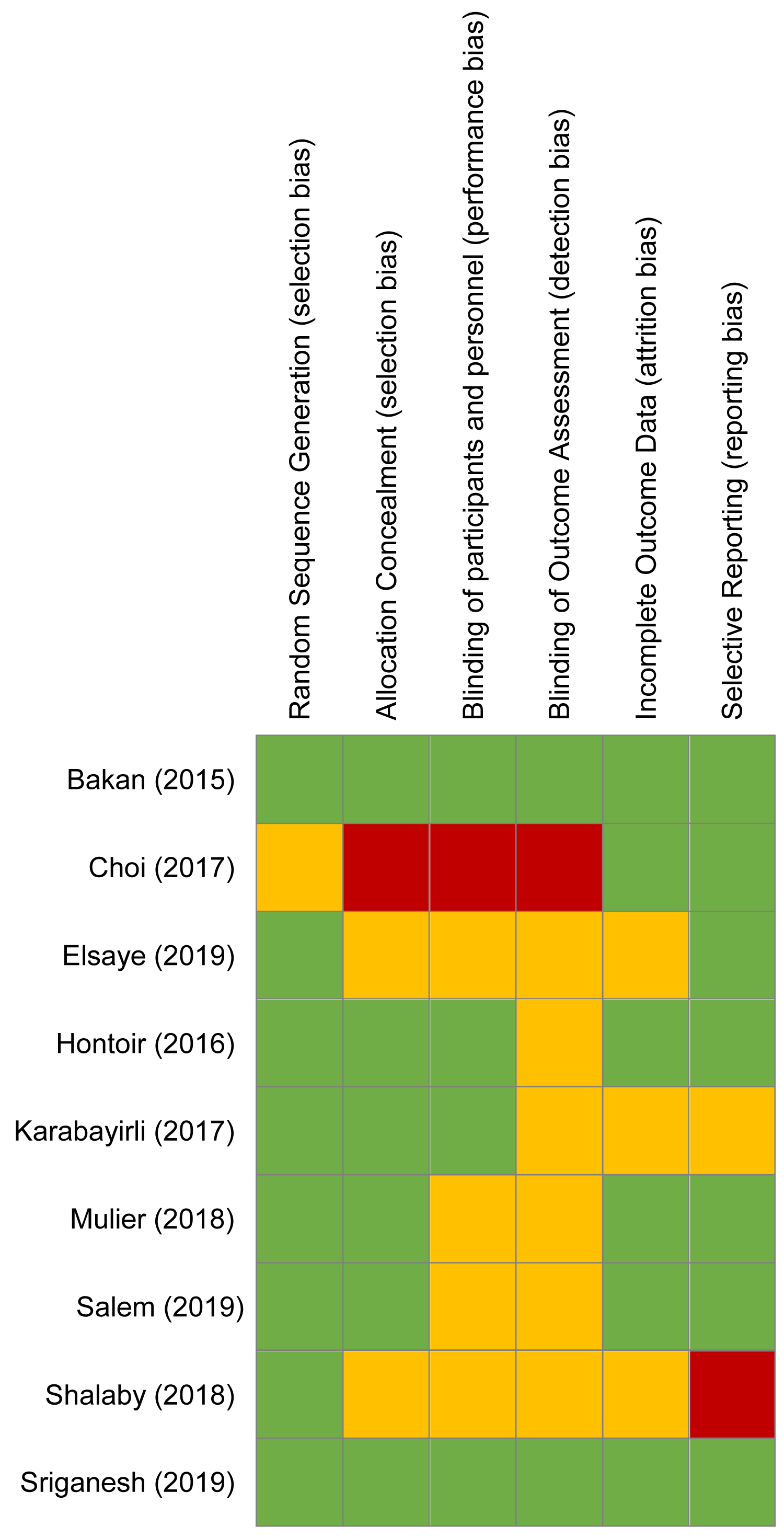 Figure 3: Cochrane Collaboration risk of bias summary of included clinical trials. Green circle, low risk of bias; red circle, high risk of bias; yellow circle, unclear risk of bias.
View Figure 3
Figure 3: Cochrane Collaboration risk of bias summary of included clinical trials. Green circle, low risk of bias; red circle, high risk of bias; yellow circle, unclear risk of bias.
View Figure 3
The characteristics of studies included in this study are shown in Table 1. All primary studies included a total of patients ranging from 40 to 80. Regarding the types of surgery, three trials included patients scheduled for laparoscopic cholecystectomy [24,27,35], two for bariatric surgery [33,36] and four for different types of elective surgeries [28,31,32,34]. Two studies included all types of surgical operations [29,30].
Table 1: Trial characteristics. View Table 1
Three trials investigated remifentanil as an intra-operative opioid regimen [28,31,32], two explored fentanyl [24,35], one sufentanil [33]; two trials compared fentanyl and remifentanil to a control group [27,34]. All included trials administered volatile anaesthetics to maintain anaesthesia except two that administered propofol [27,34].
Table 2 shows primary and secondary outcomes of interest in all included studies. In three studies, patients reported pain using a visual analog scale (VAS) score [24,28,33], two studies used the numerical rating score (NRS) to quantify pain [27,31]. Both scoring systems ranged from 0 to 10. The three meta-analysis used a standardised 0-10 analogue scale [29,30,36].
Table 2: Summary of findings. View Table 2
In two studies the first measure of pain score at rest were not reported [34,35]. Five studies also did not report pain scores at 24h postoperatively [27,32,34-36]. Furthermore, Elsaye, et al. [24] showed pain scores in the form of a graph only. Thus, numbers were extrapolated for this study as the authors did not respond.
Mean pain scores at rest in the first measure were statistically lower (p < 0.05) in opioid free anaesthesia (OFA) than in opioid based anaesthesia (OBA) in six studies [24,27,30,31,33,36] and mean pain scores at 24 postoperative hours were also statistically lower in five studies [24,27,30,31,33].
Use of supplemental postoperative analgesia was reported inferior in the OFA group in five articles, but only two of them reported statistically significant difference in the number of patients that required rescue analgesia [24,27].
Four trials found a significantly lower requirement of intravenous (i.v.) morphine consumption equivalents at 24h postoperatively in the opioid free group [30,31,33,36].
The incidence of PONV was reported in all but one study [31]. Eight of the ten studies that compared the incidence of PONV in opioid versus non-opioid groups observed a statistically significant trend toward a decrease in PONV among patients who did not receive opioids [24,28-30,33-36]. The use of antiemetic drugs was significantly lower in the opioid free group in four of five studies [27,28,34,35].
In all studies length of stay in the post-anaesthesia care unit (PACU) was longer in the opioid free group, but only three of six trials reported a statistically significant difference [27,28,30]. Finally, total length of stay in the hospital was investigated by two trials and was similar between groups [33,35].
This review investigated the effect of opioid-free anaesthesia, compared with opioid-based anaesthesia on postoperative pain, rate of postoperative nausea and vomiting and length of stay in PACU and total length of stay in hospital.
One of the three pillars of balanced anaesthesia is the administration of opioids in the perioperative period as antinociceptive agents [3,8]. However, postoperative pain remains a real problem and there is no clear evidence that intraoperative opioids result in reduction in pain scores [9]. Indeed, central nervous system sensitisation can be induced by the nociceptive response to the surgical trauma resulting in postoperative hyperalgesia [8,14]. Therefore, one would say that blocking the nociception response with intraoperative opioids, could be a solution to prevent severe postoperative pain [14].
However, postoperative pain management is a more complex system. A 2014 meta-analysis that evaluated the clinical consequences of intraoperative doses of opioid revealed that high doses of opioids during surgery are associated with an increased perception of pain and increased postoperative opioids requirements [11].
Opioid-induced hyperalgesia, also called Opioid Paradox, is a sensitization process whereby opioids, paradoxically, cause increased pain sensitivity [8,11,12]. These neuroadaptation processes cause a highlighting of existing pain and enablement of chronic pain development [12,13]. All the opioids can potentially induced hyperalgesia, particularly by short-acting opioids such as remifentanil. Furthermore, this process is dose-dependent, and the clinical relevance is most apparent in very painful procedures [14,37].
Opioid-free anaesthesia arises from the attempt to develop anti-hyperalgesic techniques to improve postoperative pain control.
This technique is based on two principles: First, opioids cause sensitization of the central nervous system and therefore their use should be minimized; second, there are other drugs with different mechanisms of action that also have good analgesic power [8,14,5,38]. Thus, by combining different drugs that act on different receptors, their analgesic effect is enhanced leading to a reduction in opioid use. This strategy helps to reduce the incidence of opioid-induced adverse effects, reduce the incidence of opioid-induced hyperalgesia and spares opioids as analgesics for the postoperative period [5,8,14,38].
A relevant meta-analysis by Frauenknecht, et al. evaluated the use of intra-operative opioids with the control being no opioids (normal saline) [29]. Although a control group with no opioid may be called OFA, it is important to clarify that the OFA technique is based on the incorporation of different non-opioid techniques as part of a multi-modal analgesia plan.
Nevertheless, Frauenknecht, et al. meta-analysis demonstrates that there is no significant difference between the postoperative pain scores and morphine consumption of the opioid-inclusive anaesthesia group compared to normal saline-treated group. In other words, this study demonstrated that opioid-based anaesthesia does not offer a significant advantage for postoperative pain outcomes [29].
The trials included in this review revealed that pain scores at rest in the first postoperative measure as well as at 24 postoperative hours were lower in the opioid free anaesthesia group when compared to opioid based group. Also, opioid-free anaesthesia when compared with opioid-based anaesthesia is associated with lower i.v. morphine equivalents requirements in 24 postoperative hours and lower request of rescue analgesia.
Grape, et al. meta-analysis demonstrated that dexmedetomidine opioid-free anaesthesia was superior to remifentanil opioid-based anaesthesia with improved pain outcomes in the immediate postoperative period and for up to 24h postoperatively, as well as, lower requirement of i.v. morphine equivalents [30]. Clonidine, also an alpha-2-agonists, was used in one trial that reported statistically lower pain scores in immediate postoperative period and 24 postoperative hours in the OFA group [31].
Singh, et al. meta-analysis regarding patients undergoing bariatric surgery concluded that patients who received dexmedetomidine required 33% less opioids in the first 24h after surgery in comparison with the controls [36].
Prolonged analgesic effect of dexmedetomidine may explain these findings. Dexmedetomidine is a highly selective alfa-2-agonist that has anxiolytic, sympatholytic, and analgesic properties. It has been used as an opioid substitute in various surgical interventions because has been shown to lower postoperative pain scores, opioid consumption, and the risk of opioid-related adverse events [39,40].
The reduction of postoperative pain by dexmedetomidine may be explained by activation of alfa-2-adranoreceptores that inhibited release of substance P from the dorsal horn, which leads to a reduction on the nociceptive inputs [28,40].
In addition to our primary outcome, opioid-free anaesthesia was associated with reduction in postoperative nausea and vomiting. Also, the use of rescue antiemetic drugs was significantly lower in the opioid free group [27,28,34,35]. Likewise, the three meta-analysis included in this review also reported significantly lower incidence of PONV in 24 postoperative hours [29,30,36].
Not only postoperative opioid use, but also intra-operative administration are risk factors for postoperative nausea and vomiting [29]. While PONV is considered an unpleasant but inherent effect of opioid-based general anaesthesia, patients ranked vomiting as the main outcome to be avoided in postoperative period, ahead to postoperative pain [41]. PONV is responsible for system resource consumption including prolonged length stay in both recovery area and hospital and finally, increased costs of health service [29]. The risk factors that affect the incidence of nausea and vomiting are multifactorial and include type of anaesthesia, type of surgery and characteristics of the patient [42]. Therefore, an opioid-free regimen should be considered, especially in high-risk patients, among the strategies to prevent PONV.
However, some trials provide no information on routine prophylaxis against PONV or rescue antiemetic drugs [24,31,33,36]. It is not clear whether the increased incidence of PONV in the opioid based group can be wholly attributed to opioid administration versus lack of prophylaxis.
The length of stay in the post-anaesthesia care unit (PACU) was longer in the opioid free group, but only three studies presented statistically significant results [27,28,30]. Total length of stay in the hospital was only investigated by two trials and was similar between groups.
Sultana, et al. claims that systemic alpha-2-agonists do not prolong recovery times [15]. Nonetheless, many trials reported a statistically longer stay in post-anaesthesia care unit in the opioid-free group [27,28,30]. This is related to the fact that dexmedetomidine has a long half-life (2-2.5 h), thus associated with slow recovery [27,37,39,40].
There are notable limitations to this review. Firstly, our review was based on a limited number of studies, most of which had inherent biases. Secondly, the studies included present a large heterogeneity. Consequently, it was not possible to form subgroups regarding the intraoperative opioid regimen, maintenance medication and type of surgery for further analysis. Finally, we were unable to draw any robust conclusion regarding the impact of opioid-free anaesthesia on total length of stay in the hospital. Therefore, the existing literature would benefit from additional trials to better define the impact of each anaesthetic strategy on health system resources.
Despite the current trend in favour of individualised anaesthesia modalities and the growing number of providers practicing opioid-free anaesthesia, there is astonishingly little data. There are specific populations that benefit from the use of OFA, namely in opioid addiction, chronic pain syndromes, morbid obese patients, obstructive sleep apnoea, cancer surgery and abdominal surgery. However, contraindications for the use of OFA are less clear.
There is evidence that opioid-free anaesthesia, when compared with opioid-based anaesthesia, does not present inferior results regarding pain scores or opioid consumption in the postoperative period. It is also associated with reduced postoperative nausea and vomiting. However, many drugs used in this technique such as ketamine and gabapentin also have substantial addictive potential and may also lead to long-term difficulties [38].
The opioid-free anaesthesia presents as future challenges an objective documentation of both its short-term and long-term benefits and inconveniencies using large sample sizes and development of adequate monitoring of intraoperative nociception. In order to clarify some questions, the Postoperative and Opioid-Free Anesthesia (POFA) trial, a prospective randomised, single-blind, multicentre study is now ongoing (NCT03316339), recruiting 400 patients [43]. The results will give information regarding safety of opioid-free anaesthesia technique.
In conclusion, further research with robust methodological trials with large sample sizes are required to better determine the efficacy and safety of opioid-free anaesthetic strategy.
The authors declare no conflicts of interest.
The authors have no sources of funding to declare for this manuscript.