The purpose of this investigation was to identify causes and triggers of air entrainment related to intravenous (IV) access in a setting of clinical, operative or interventional procedure.
An observational prospective trial was conducted in operating room settings across multiple study sites. A total of 120 surgical patients undergoing a variety of interventional and surgical procedures were included, representing multiple patient populations, case types and anesthesia setups. The ClearLine IV, a device that detects and removes air from IV tubing, was inserted into the IV circuit. Clinicians followed standard protocols. Blood warmers were used at the practitioner's discretion. IV fluid was administered at a constant flow rate or through a bolus given over 30-60 seconds per standard of care. The volume and duration of fluid administration was recorded, along with the frequency, duration and amount of air captured by ClearLine IV.
Data confirmed a 52% probability of having air in the IV tubing, 79% when using a fluid warmer or bolus, and for every 10 mL of blood products administered, the risk increased by 0.5%. The baseline probability of dense air (defined as greater than 1000 µL per L of fluid) increased from 0% to 20% with the inclusion of a syringe setup, and up to 50% with a warmer or bolus.
Air entrainment occurs in the presence of an IV in the hospital setting, and air burden, the amount of air captured, is increased by use of a fluid warmer, administration of medication by bolus, delivery of blood products, and inclusion of IV syringe setups.
There is widespread acceptance that air in intravenous (IV) fluid tubing lines can pose a significant risk to patients [1]. In fact, air embolism is second on the Centers for Medicare and Medicaid list of Hospital Acquired Conditions (HAC), yet it remains elusive to combat in practical, everyday clinical environments [2]. The knowledge of the quantity of air, the air burden, typically administered to patients in the operating room and the source of the air has received little study. With research surrounding the effects of microbubbles on vascular endothelium and glycocalyx, there is mounting concern for the unknown harm air causes patients in everyday clinical practice. Understanding the sources of air in IV tubing is the first step in determining the best preventative measures to reduce air burden for patients.
Present knowledge around air embolism focuses on arterial embolism, specifically in catastrophic events, while research on smaller amounts of venous air emboli is scarce. The theory that small venous air emboli are innocuous is controversial since the amount of air required to cause symptoms and catastrophic events depends on many patient and environmental factors [3-5].
Venous air embolism (VAE) may originate from IV placement and use, central venous catheter placement, use and removal, hemodialysis, trauma, surgical and diagnostic procedures, and surgeries with patients in the sitting or beach-chair position [6]. Air entering the venous vasculature through IV tubing during routine clinical procedures is not well described. Routine air presence in IV tubing occurs due togas coming out of solution during the use of fluid warmers, and may occur during pressure infusion [7-9]. Another source of air in IV tubing includes syringe boluses of medication and fluid where a mean of 0.02 mL of air is injected [10]. Bolus injections may introduce air from the syringe, stopcock, or push through microbubbles present in the IV tubing due to changes in fluid temperature.
In cases of large VAE, the filter capacity of the pulmonary capillary bed may become overwhelmed, allowing air to translocate to the arterial circulation. Known as a paradoxical embolism, this phenomenon is more common in patients with a patent foramen ovale (PFO) that allows direct air passage from the venous to arterial system [11,12]. With up to 25% of the general population having a patent foramen ovale (PFO), and many undiagnosed, this risk of air intrusion raises clinical concern [6,13-15].
In infants, intravenous injection of small amounts of air with each syringe can accumulate and result in fatality [16]. Evidence suggests that even microbubbles, defined as air bubbles less than 1000 µm in diameter, that enter the vasculature can cause harm [17]. As the microbubbles enter circulation, they may cause damage to glycocalyx, a glycoprotein on the endothelial cell surface [18]. By increasing cell permeability, this damage may decrease cell viability and increases cell apoptosis [19]. Small venous emboli cause tissue ischemia, an inflammatory response and complement activation [5]. Following cardiac surgery, neurological complications have been potentially linked to microemboli and include lowered consciousness, seizures and cognitive impairment [20]. While conclusive evidence of the harm of microemboli is yet to be established, strategies to understand and reduce air entrainment are important in clinical practice [21].
The objective of this study was to determine the extent and frequency of the clinical manipulations that lead to air presence and potential areas for improvement in preventing air from reaching the patient's vasculature. The knowledge gained from the study is intended to identify factors that place patients at higher risk of air entrainment during routine IV procedures, and to inform clinicians on this risk of increased exposure to venous air during surgical procedures. The authors hypothesize that air presence in the IV tubing, and ultimately the circulation, is caused by specific medical interventions.
A multicenter, prospective observational study was conducted at three United States academic institutions in accordance with Good Clinical Practice standards under the oversight of an Institutional Review Board (IRB). In two out of three centers, patient consent was required and obtained from all patients, while in one center the IRB waived the requirement for a study-specific informed consent due to the commercial clearance status of the ClearLine IV system (ClearLine MD, Woburn MA, USA) and observational nature of the study. Patient confidentiality was established, and names were replaced with identification numbers for the purpose of data collection, analysis and reporting. The study was not registered in an applicable database before enrollment began as it was unclear if the study met the requirements for registration. The sponsor registered the study after completion and after review of the Checklist for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (ACT) Under 42 CFR 11.22(b) for Clinical Trials Initiated on or After January 18, 2017 which became available in June 2018 (NCT03723408).
Study sites were provided with ClearLine IV devices that contained a memory card for recording every event of air detection during the procedures (Data Monitor device), along with study-specific data collection tools to record procedural information. The ClearLine IV device consists of a control unit and a sterile disposable component. The device is connected to an infusion line and uses ultrasonic sensors to detect in-line air. The sensor rapidly sends short bursts of low power ultrasonic energy into the tube. If the tube is full of liquid then the ultrasonic energy travels through the tube and liquid and is detected by a receiver on the other side. However, if there is a gas bubble in the tube, some ultrasonic energy is reflected off of the gas-liquid or gas-tube interface and less energy is received by the sensor. The sensor is calibrated with bubbles of known sizes in-factory to ensure that only gas bubbles of a specified size are detected. Once detected, the device diverts fluid flow to a waste collection bag and a second ultrasonic sensor detects when the air has been removed causing the controller to return intravenous flow back to the patient's infusion line. As such, the device does not impede the IV fluid flow. Bench testing to evaluate fluid loss per air removal cycle found that when the flow rate was 600 mL per minute, a maximum of 10 mL of fluid volume was lost. The minimum detected air volume is 25 µL. The Data Monitor device included in the ClearLine IV for study purposes, consisted of hardware and software that are independent, and did not affect operation of the ClearLine IV device. The Data Monitor resided in the battery pack area of the ClearLine IV and collected objective data to measure: 1) The amount of air removed from the IV tubing by the ClearLine IV; 2) The frequency and duration of ClearLine IV mechanism activity; and 3) The occurrence of any alarm conditions in the ClearLine IV.
The method of ultrasonically detecting air masses 25 microliters or greater is proven to be reliable through verification and validation of the ClearLine IV, as well as the ultrasonic sensors. A precise data set was collected which allowed the calculation of the air volume which was administered through the IV line. One of the key variables in this computation is fluid velocity. Fluid velocity was recorded during the procedures, as well as time of bolus activity, in order to allow the correct air volume calculation to occur. Inaccuracy of this reported flow rate would be the major source of any inaccuracy of the air volume calculations.
Recruitment was conducted between September 2016 and January 2017. Patients scheduled for procedures were assessed for eligibility. All patients included in this analysis met the prospective clinical inclusion and exclusion criteria. Patient inclusion and exclusion criteria targeted both pediatric and adult patients with no age restrictions, a minimum weight of 5 kg, and who were scheduled for a prospectively identified list of surgical or cardiac catheterization procedures allowing for the collection of a minimum of two hours of procedure time. Basic patient demographics at time of the procedure were collected on enrolled study patients. All eligible patients were assigned to the intervention. Six subjects were excluded from the analysis due to missing data making it impossible to align the operating room times with the air noted by the device. See Figure 1 for details of enrollment and analysis.
 Figure 1: TREND flow chart of enrollment details.
View Figure 1
Figure 1: TREND flow chart of enrollment details.
View Figure 1
ClearLine IV was connected in-line with the IV circuit in interventional and surgical patients undergoing the procedures listed in Table 1. The device was set up by operating room staff in conjunction with the anesthesia team and was used throughout the duration of the procedure. The patients and staff were not blinded in this study. IV fluid was administered at a constant flow rate with bolus fluid medications given over 30-60 seconds. Blood warmers were used at the discretion of the practitioners. Fluid administration was recorded by noting volume and duration of administration. Throughout each procedure, the start and stop time of the device, fluid administration and medication boluses were documented along with fluid types, fluid volume and the time fluids were administered. At the end of patient enrollment, the memory cards were collected. A case report form (CRF) was completed by clinical site personnel to collect information on IV fluid delivery and relevant details of the type of procedure, including the timing of clinical events and fluid flow rates. Using the CRFs citing times of clinical manipulation of the IV and the air mass removal recorded on the memory card, the air masses were correlated to clinical events and the case breakdowns were generated Figure 2.
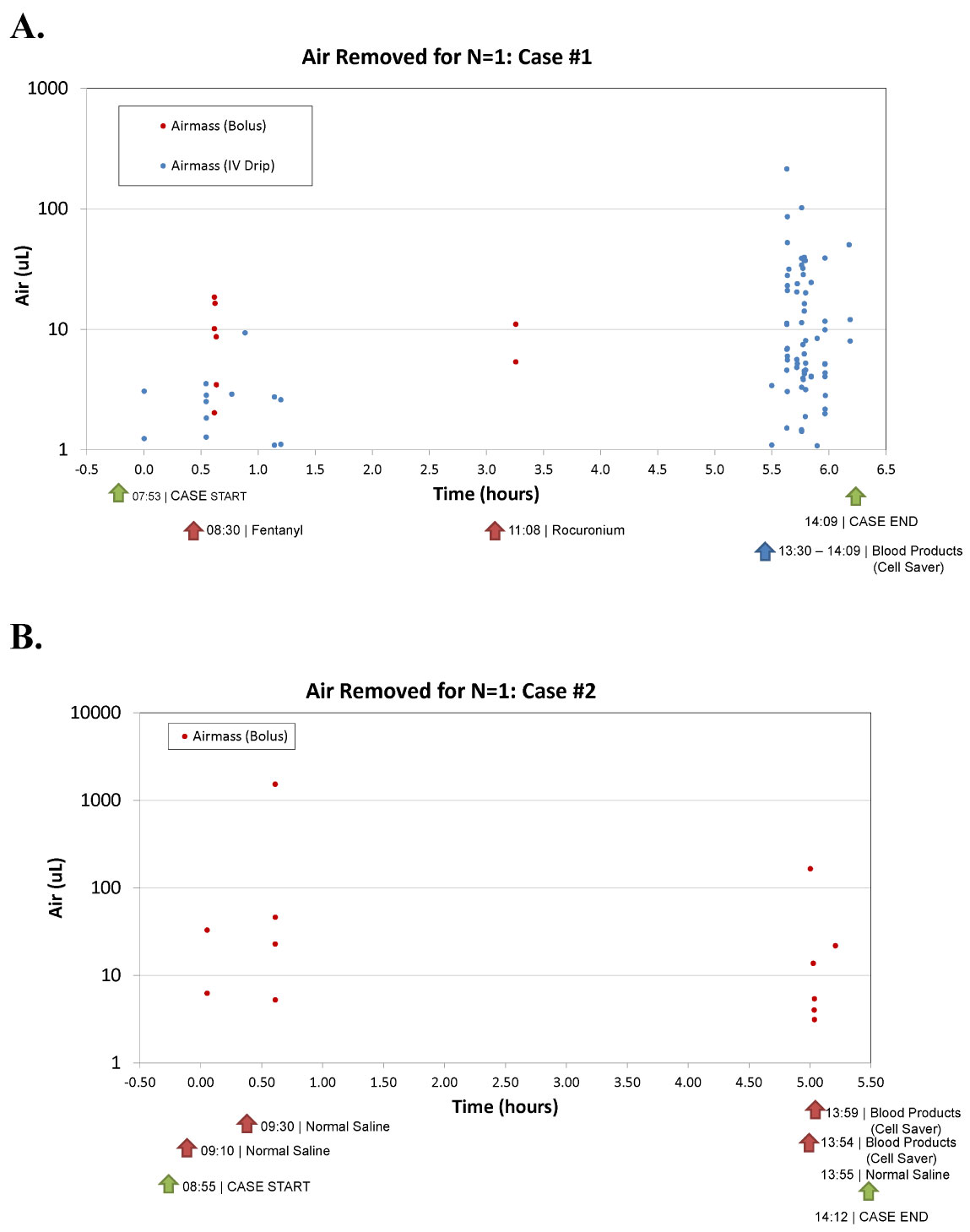 Figure 2: Case breakdowns.
View Figure 2
Figure 2: Case breakdowns.
View Figure 2
Table 1: Demographics and Procedural Characteristics (N = 120). View Table 1
Clinical centers followed their standard of care in terms of fluid/blood warmers. When used, warmers were placed above the device, with the line exiting the fluid warmer entering the ClearLine IV. Clinical centers had the option of using air filters below the ClearLine IV. Where multiple lines were used in patient cases, the ClearLine IV was used in one line. ClearLine IV was only used in IV lines without infusion pumps, as these create an undesirable source of variability. Per protocol, investigators followed their routine practice for air bubble detection and removal below the ClearLine IV cartridge and the patient. Figure 3 details the ClearLine IV device setup for each type of fluid circuit encountered in the study.
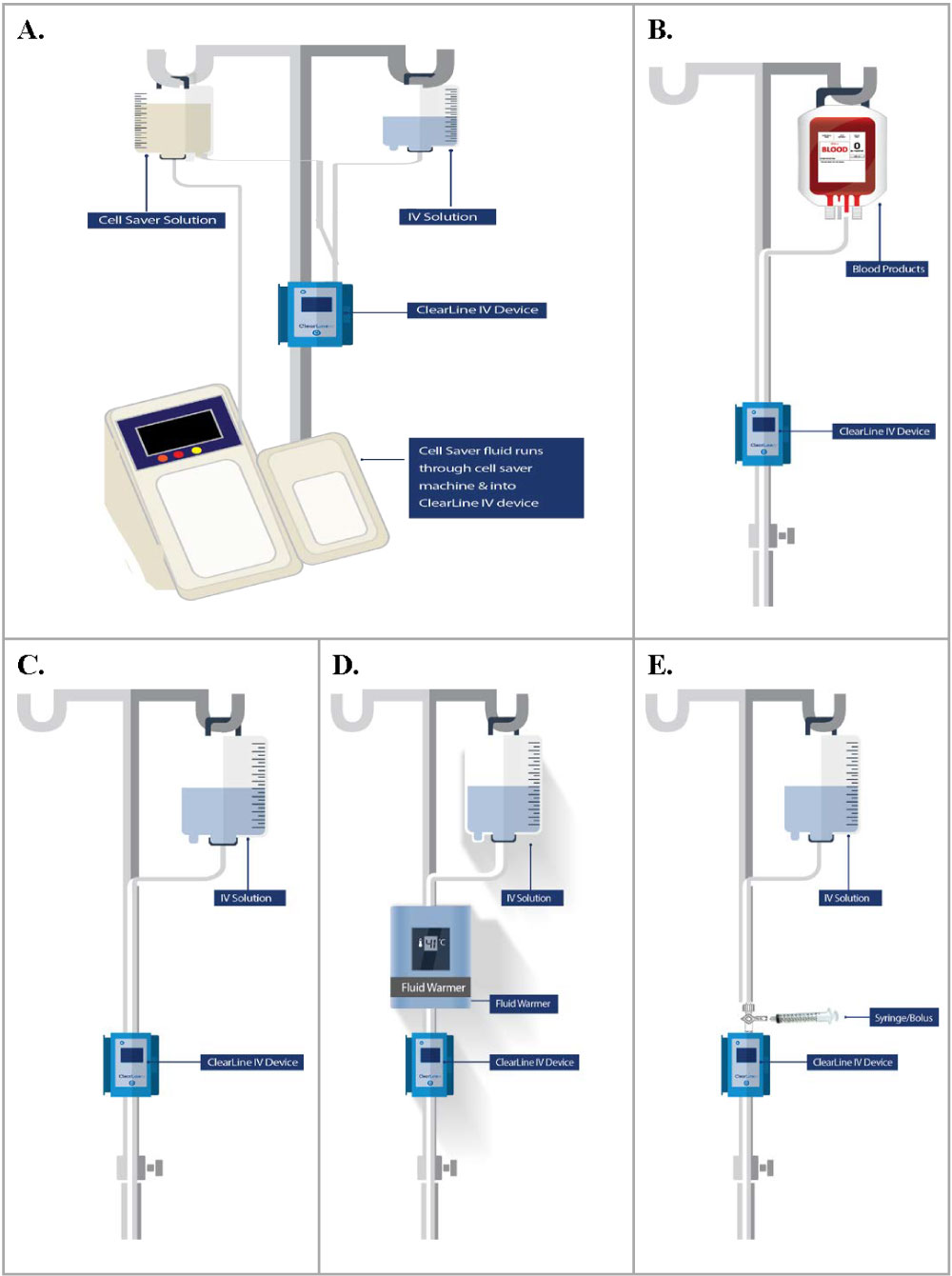 Figure 3: ClearLine IV setup in-line with the fluid circuit.
View Figure 3
Figure 3: ClearLine IV setup in-line with the fluid circuit.
View Figure 3
Data were analyzed to assess the primary goal of determining how various factors influenced the presence and volume of air in the IV. Factors considered were the volume of blood products, the use of a warmer or the administration of a bolus, and the use of a syringe or a drip setup. Two sample t-tests were performed on populations characterized by the two binary factors to evaluate if their respective means significantly differed in volume of air (uL/L) detected in the IV tubing. In the case of blood products, a non-binary factor, a non-parametric rank-correlation was performed to assess if there was a trend associated with increased blood products and increased air.
The interactions between clinical factors were evaluated via logistic regression. Variables were included in the regression model with the threshold for stepwise selection at a P < 0.1 level of significance. Binary indicators on each case were computed for the presence of any air and of dense air which was defined as a volume of air greater than 1000 µL per L of fluid. These binary indicators served as response variables in the logistic models. A 5-fold cross validation was used to ensure that the logistic models were not over-fit. Area under the receiver operating characteristic (ROC) was computed to validate logistic models. From the estimated regression coefficients, the computed probabilistic risk was estimated for each of the factors considered in the models using logistic transformation.
A total of 120 patients underwent the study procedures in the operating room or cardiac catheterization laboratory. A summary of patient demographics and procedure types is included in Table 1 and Table 2.
Table 2: Procedure Type (N = 120). View Table 2
Procedure time ranged between 1 and 9.6 hours (mean 4.1 hours). A warmer was utilized in 90 (75.0%) of the study procedures; (42.5% Hotline® Blood and Fluid Warmer (Smiths Medical ASD, Inc., Rockland MA, USA), 19.2% Enflow® IV Fluid and Blood Warming System (CareFusion, Vernon Hills IL, USA), 13.3% 3M™ Ranger™ Blood and Fluid Warming System (3M HealthCare, Neuss, Germany). IV fluids used in conjunction with the ClearLine IV included Lactated Ringers (20%), Normal Saline (65.8%) and Plasmalyte (15%). Blood products were delivered through the ClearLine IV in 63% of procedures. Drip lines were utilized in most of study procedures (80%); with a syringe setup in 20% of cases. The number of IV bags used ranged between 0 and 6 bags (mean 1.5 bags), with an overall IV fluid volume of 132.45 liters. No device-related complications or complaints of malfunction were reported throughout the study. In one case there was a protocol deviation in which the ClearLine IV was inadvertently turned off mid-procedure but then restarted without incident. This event was factored into data analysis. A ClearLine IV device alarm sounded in one procedure alerting the clinician that the device door was open.
Case breakdowns demonstrated that clinical manipulations such as warmer usage, blood product delivery, bolus injections and syringe setup correlated to specific air events captured by ClearLine IV. Figure 2 shows two examples of case breakdowns from two separate facilities that display each air mass as a bullet point and time-stamped with corresponding procedure events. Both highlight the variables determined to be markers for air intrusion.
Figure 4 displays the number of cases with each variable and the corresponding number of those that contained air. In total, 98 out of 120 (81.7%) cases resulted in air being introduced to the patient.
 Figure 4: Cases broken down by variable and air burden.
View Figure 4
Figure 4: Cases broken down by variable and air burden.
View Figure 4
Certain factors significantly altered the volume of air in sub-populations in the study. An array of both combined and individual categorical variables was considered to classify patients into subpopulations. The variables were selected from the binary variables recorded in trials, as well as the bases of whether the variables and the resulting combinations thereof seemed as though they would be correlated with air in the line. Table 3 lists the results of the statistical tests ordered by significance. The use of a warmer or bolus administration was the most significant (p = 0.005), followed by the syringe drip (p = 0.066).
Table 3: Clinical factors associated with the presence of air detected in the IV. View Table 3
The non-parametric correlations showed a positive trend, significant at the 5% level, between increased blood product administration and increased air in the IV with a spearman's rho of 0.204. Logistic models performed well considering the small derivational data set and the simple combination of predictors. Using the significance-based exclusions, the dense air model retained the warmer or bolus and syringe or drip variables whereas the any-air model selected the warmer or bolus, syringe or drip, and the blood products volume variable. Figure 4 highlights that 80 out of 90 cases (88.9%) using a warmer contained air. The most significant predictor of air is use of a warmer or bolus, with the probability of air entrainment at 79% compared to a baseline of 52% Figure 5.
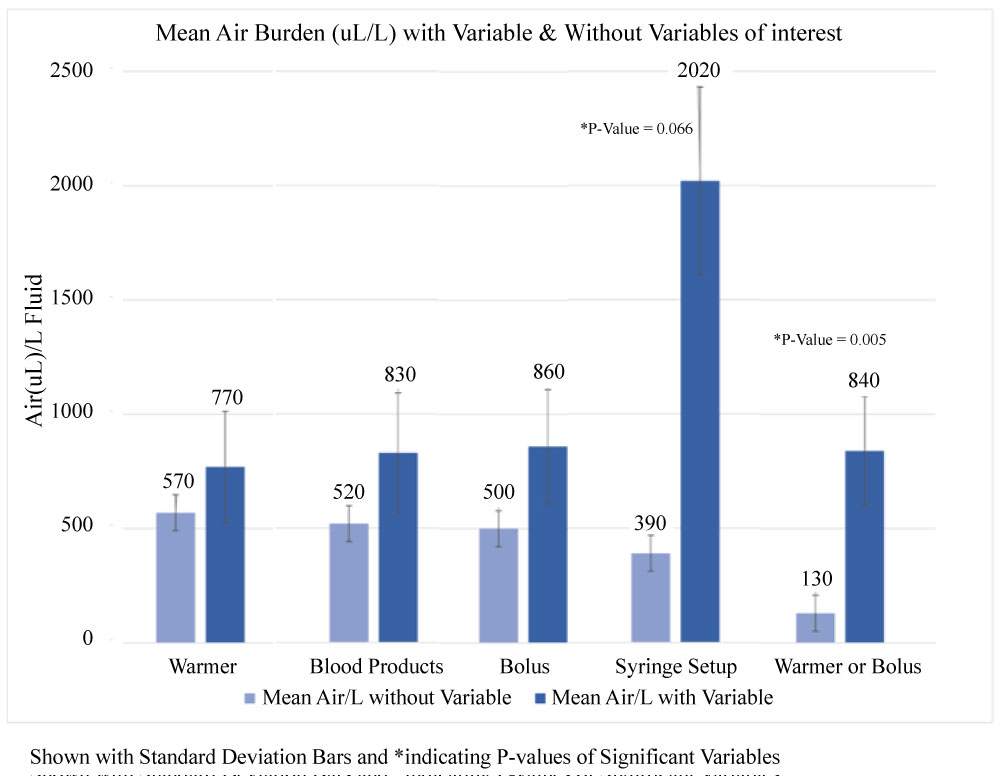 Figure 5: Mean air burden (uL)/L with and without variables of interest.
View Figure 5
Figure 5: Mean air burden (uL)/L with and without variables of interest.
View Figure 5
C-Statistics for the models predicting any air in the IV circuit were 0.80 [0.90, 0.70] without 5-fold cross validation, and 0.79 with 5-fold cross validation. C-statistics for the models predicting dense-air were 0.74 [0.60, 0.87] without 5-fold cross validation and 0.65 with 5-fold cross validation. Cross validated c-statistics falling within the initial confidence bounds suggest that the prediction models are stable.
Upon conversion of the logistic models to probabilities, the warmer or bolus variable indicates the highest increase in expected probability for both the presence and the volume of air in the IV circuit. In the any-air model, an unparameterized blood products variable lends an approximate 0.05% increase in risk per mL of blood products administered and having a warmer or bolus lends an additional approximate 17%. The baseline probability for having any-air in the IV circuit is estimated to be 52% with probability of any air in conjunction with a warmer or a bolus is 79%.
In the dense air model, the syringe variable adds an approximate 20% increase, from an estimated baseline probability of 0%. The presence of a warmer or bolus results in a 50% estimated likelihood of having dense air.
Throughout the study, use of fluid warmers resulted in a consistent correlation to air entrainment. Looking at case breakdowns across patient populations and study sites that used fluid warmers, air occurred in clusters in an unevenly distributed pattern. For example, Figure 2A depicts a cloud of air masses, particularly when the cell saver blood is given to the patient. Air entry is not consistent small volumes, and instead is intermittent and massed together, potentially creating greater chance of harm.
The purpose of the study was to determine the extent and frequency of venous air burden in patients in an operating room setting, the clinical manipulations that result in air entry, and potential areas for improvement in preventing air from reaching the patient. With the broad patient population included, the variety of procedures involved, intraprocedural differences, and small sample size, the statistical analysis showed many of the variables were not significant, however some became strong predictors in the regression model. A larger and more comprehensive study should follow and look specifically at each trend.
In this study, the amount of air removed from IV tubing and the frequency and duration of air was measured using the ClearLine IV System. Utilizing standard clinical techniques in adult and pediatric operating rooms, the data captured by ClearLine IV represents typical characteristics of air in IV tubing across patient populations and case types. While we cannot discount the possibility that knowledge of the study and the presence of the ClearLine IV device impacted clinical practice in these study cases, key trends were witnessed and correlated with previously published data regarding air generation from warmers and syringes [7,14,20].
The data identifies specific clinical manipulations that generate air increasing the patient's air burden; cases with fluid warmers, bolus injections of medication and fluid, syringe IV setups, and administration of blood products. Specifically, when either a warmer or bolus is used, there is a high correlation of air introduced (P = < 0.001) with a probability of 79% of any volume of air entering IV tubing. The use of warmer and bolus administration in combination resulting difference in means was significant with 95% confidence.
Examining the data by individual cases, the triggers of air introduction are evident. Looking at Figure 2A, air masses are removed in clusters, correlating to specific events. For instance, 8:30 AM a bolus is given with subsequent air masses, 11:08 AM another bolus given with air masses, and at 13:30 cell saver blood is given with many air masses following. These patterns were observed across study sites, patient populations and procedures. Figure 2B represents a pediatric patient utilizing a syringe IV setup and shows air masses following the medication/fluid injections. These results demonstrate that manipulation of IV tubing resulted in air entrainment.
Specific precautions surrounding medication injections via syringe should be taken into consideration. In this data set, cases of air in the IV tubing increased when boluses of medication were given. Figure 2 cases both correlate air mass in the IV tubing with medication and fluid injections. This directly relates to Figure 5 showing the statistical calculation of significance for syringe and warmer or bolus. Every time a syringe is connected to the IV, there is a probability of air entry. Specifically, in the dense air model (defined as greater than 1000 µL per L of fluid), the predictability of air in the IV tubing increases from a baseline of 0%, to 20% in syringe setups, and up to 50% in warmer or bolus cases. This is particularly alarming for small infants and children who do not receive much fluid throughout a procedure and are given small doses via syringe. The risk of right-to-left shunting is high in the neonate congenital heart population, offering an avenue of venous air entering the arterial circulation.
The population in this study was very diverse in age as well as procedure, although most patients were undergoing cardiac bypass surgery (60.8%). Based on these results, a predictive model of air entrainment with a large, specific population would be an interesting follow-up study. With the findings described here, one can focus on a variable (warmer, blood products, syringe setup, bolus) and analyze how accurate these trends are in determining air entrainment in IV tubing. The next step would be to determine the predictability of the volume of air entering IV tubing.
This study documents specific clinical manipulations causing air entrainment in patient IV tubing in a broad patient population including all ages and a variety of procedures. Without intent or knowledge, clinicians may subject patients to a preventable air burden in everyday practice. Particularly in instances of fluid warmers, blood product delivery, bolus injections, and syringe setup cases, patients have an elevated risk of air entry into IV tubing. Although the amounts of air detected in this study were overall low and below those anticipated to cause harm in the venous vasculature, patients with a PFO have an increased risk even with relatively small amounts of air. By understanding the triggers and predictability of air entry, clinicians can take steps to increase vigilance and be proactive to reduce the air burden. Currently, pumps often alarm when air is detected leading to manual intervention, although recent recalls have called the efficacy and reliability of these alarms into question. The device used in the present study automatically detects, captures and eliminates air from IV tubing reducing air burden to the patient.
In conclusion, the results of this study suggest that multiple factors increase air burden during routine clinical procedures. As the amount of air required to cause patient harm is highly variable, the results of this study suggest that any additional step to reduce air burden may increase patient safety, particularly in cases where a warmer or syringe setup is used, bolus medication is administered, and where blood products are administered. Further studies in a larger population could be performed to demonstrate the impact of the device on rates of complications related to air embolism.
The authors would like to acknowledge Jerra Lobozzo for her support with the conduct of the study and editorial review of the manuscript.