Perineural invasion (PNI) refers to the infiltration of tumour cells into the perineural space of a nerve. It is predominately associated with cutaneous squamous cell carcinomas (cSCC). While PNI has a low level of incidence, it significantly affects the prognosis leading to a poorer outcome. We report a rare case of extensive PNI from a scalp cSCC in a Caucasian gentleman. The supraorbital, supratrochlear and optic nerves were involved requiring an orbital exenteration as part of the surgical management of the cSCC. This is a unique case where a distant cSCC resulted in perineural and intraneural spread to all three of these nerves upto the orbital apex and there are no previous reports of this in the literature.
Perineural invasion (PNI) is defined as a histopathological finding of malignant cells within the perineural space of a nerve [1]. It has a more aggressive course, with risk of superficial or intracranial spread, and holds a greater risk of recurrence. PNI affects approximately 2.6% of primary cutaneous squamous cell carcinomas (cSCC) and has a greater incidence in recurrent tumours [2]. PNI is therefore an important predictive factor of prognosis in high risk cSCCs in the head and neck and staging of the cancer is essential for directing its management [3]. In recurrent cSSC that have been treated with radiotherapy or chemoradiotherapy treatment, determining the diagnosis and staging can be an additional challenge due to the difficulty in differentiating between radiation induced reaction and recurrent cancer [4]. This can result in delays in diagnosis and therefore, increase the potential for perineural, intraneural or perivascular spread.
A 76-year-old Caucasian male presented with a 20 mm × 10 mm crusted non-healing lesion on the left frontal area (Figure 1). The patient had been referred by his GP and his main presenting complaint was pain around his left eye. He had lived in South Africa before moving to the UK two years ago, where he had been treated for the cSCC and had undergone multiple skin grafts followed by a course of adjunctive radiotherapy treatment. The area had never fully healed. The patient was fit and well and the only relevant medical history was that he had developed ophthalmic shingles on the left side 7 years previously.
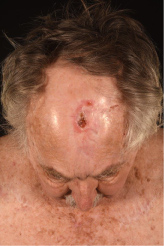 Figure 1: Pre-operative clinical photo showing the cSCC on the left frontal area.
View Figure 1
Figure 1: Pre-operative clinical photo showing the cSCC on the left frontal area.
View Figure 1
An excisional biopsy of the lesion was performed. The histology confirmed poorly differentiated invasive squamous cell carcinoma. All margins were clear, with a minimum deep margin of 3 mm; however, there was evidence of perineural invasion, and the lesion was therefore staged as pT3 N0 M0. Following discussion in the skin cancer MDT meeting, the patient was initially offered post-operative radiotherapy; however, on further review of the radiotherapy fields from South Africa, this was not possible.
The patient developed a further recurrence on the forehead as well as a nodule on the medial left eyebrow. He also complained of a numb scalp on the left side up to the vertex. Biopsy of the latter confirmed perineural invasion of the supratrochlear nerve.
Imaging (Figure 2 and Figure 3) confirmed perineural spread of tumour from the scalp all along the supraorbital and supratrochlear nerves to the superior orbital fissure. Following discussion, the patient opted to proceed with surgery which involved excision of forehead and scalp skin up to the vertex of the scalp and exenteration of the orbit. Histopathology confirmed cSCC with perineural and intraneural invasion of the supraorbital, supratrochlear and optic nerve all the way to the orbital apex. The surgical defect was reconstructed with local flaps and a dermal regeneration template (Integra) (Figure 4).
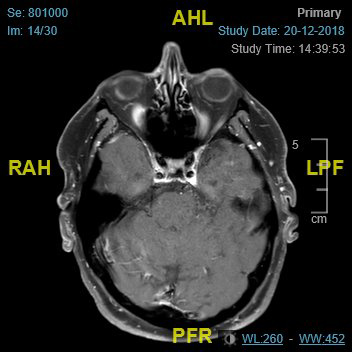 Figure 2: MRI-axial view.
View Figure 2
Figure 2: MRI-axial view.
View Figure 2
 Figure 3: MRI-coronal view.
View Figure 3
Figure 3: MRI-coronal view.
View Figure 3
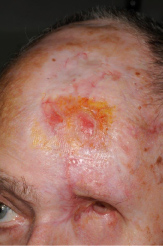 Figure 4: Post-operative clinical photo showing the surgical defect after healing.
View Figure 4
Figure 4: Post-operative clinical photo showing the surgical defect after healing.
View Figure 4
To our knowledge this is the first case of extensive peri-neural invasion from a scalp tumour, invading the orbit all the way to the orbital apex, and there are no cases reported in the literature to date concomitantly involving the supraorbital, supratrochlear and optic nerves [5]. The perioperative diagnosis of PNI is challenging as traditional, non-focused CT and MRI imaging have low sensitivity for PNI. Where histopathology indicates PNI, a focused MRI is recommended with thin slices along the course of the affected cranial nerve. Negative imaging can mislead the management in such cases, since the disease process itself is rare [6].
This patient had numbness in the distribution of the supraorbital and supratrochlear nerves. Whilst pain, anaesthesia, dysesthesia, and/or paraesthesiamay be common symptoms in PNI, most patients are asymptomatic. Patients with clinical symptoms in PNI of cSCC generally have a poorer prognosis [7].
This patient developed perineural and intraneural invasion in the same distribution as the previously clinically diagnosed ophthalmic shingles. Whilst the shingles occurred 7 years ago, this case report raises the question of whether this had any relevance to the subsequent PNI. Typically, any neuralgia associated with shingles resolves in 4 weeks, but the virus can remain latent for a number years. The oncogenic potential of the varicella zoster virus has been recognised in studies carried out on animals and it is thought to have a similar effect in humans. This case compounds this preliminary research. It may be beneficial to take an early biopsy of suspicious lesions to enable a histopathological diagnosis and aid further research [8].
The histology result from the surgery carried out in South Africa was not available, which may have been crucial to planning the surgery as well as the choice of perioperative investigations. Unfortunately, because of previous radiotherapy for recurrent cSCC, further radiotherapy to this area was not possible and therefore radical surgery was required for the management of the advanced PNI. Treatment for PNI varies considerably depending on the type of malignancy, its location, the PNI distribution and extent. A study by Gupta, et al. suggested that surgery, preferably with clinical margin control, together with definitive or adjuvant radiotherapy remains the best treatment strategy for PNI, however, further high-quality evidence is needed [9].
Overall, this case highlights the need for having a high index of suspicion to enable a timely diagnosis of PNI as symptoms can insidiously develop over a number of months or years prior to any significant signs of PNI. It also demonstrates that symptomatic PNI can occur despite seemingly clear-margin excision of the malignant lesion. It is therefore recommended that a focused MRI is used to enable closer examination for suspected PNI to avoid delays in the diagnosis.
The authors declare that there is no conflict of interest.