Recurrent stress-induced cardiomyopathy with variable regional location of left ventricular dyssynergy is an unusual clinical presentation and may allow potential insights into the pathophysiologic mechanisms underlying the development of the variable subtypes of stress induced cardiomyopathy.
This case report reveals a patient with reverse Takotsubo cardiomyopathy and apical Takotsubo cardiomyopathy within a three-week period. The discussion explores the potential pathophysiologic mechanisms responsible for the variable left ventricular regional involvement in stress-induced cardiomyopathy.
Stress induced cardiomyopathy, Reverse Takotsubo cardiomyopathy, Apical Takotsubo cardiomyopathy, Sigmoid septum, Catecholamines, Non-obstructed epicardial coronary vessels
A fifty-four-year old female presented to the hospital with a complex constellation of symptoms and physical examination findings including fever, chills, and bloody diarrhea. She also was experiencing chest pressure, shortness of breath and diffuse abdominal pain. Symptoms started three days prior to admission. Upon admission to the hospital the patient was taking amiodarone 200 mg per day and vitamin D supplementation. The patient had not been exposed to direct administration of catecholamines or medications causing catecholamine surge.
Examination revealed a blood pressure of 100/50 mmHg and a sinus tachycardia at a rate of 112. Jugular venous pressure was flat and carotid upstroke was low pulse volume. She had a 2/6 systolic ejection murmur and a third heart sound. Lung examination revealed bilateral basilar crackles. There was no peripheral edema.
Past medical history was pertinent for normal coronaries in 2015, paroxysmal atrial fibrillation S/P ablation, and laryngeal cancer S/P laryngectomy with a tracheostomy. She also had a history of spinal lymphoma treated with radiation.
ECG revealed sinus tachycardia and non-specific ST changes (Figure 1). The echocardiogram revealed a left ventricle (LV) with a sigmoid septum and depressed LV systolic function (LVEF = 35%). Regional wall analysis revealed a hyperdynamic LV apical function and dyssynergy of the base (Figure 2 and Movie 1).
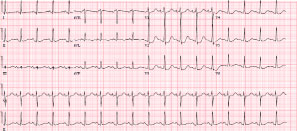 Figure 1: Sinus tachycardia with non-specific ST changes.
View Figure 1
Figure 1: Sinus tachycardia with non-specific ST changes.
View Figure 1
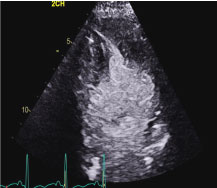 Figure 2: Apical 2 chamber end-systolic still frame revealing apical obliteration and basal akinesis.
View Figure 2
Figure 2: Apical 2 chamber end-systolic still frame revealing apical obliteration and basal akinesis.
View Figure 2
Movie 1: Echocardiographic Apical 2 chamber view of the left ventricle with contrast revealing left ventricular hyperkinesia (red arrow) and left ventricular basal dyssynergy (yellow arrow). The echocardiographic features represent the subtype of stress-induced cardiomyopathy called reverse Takotsubo cardiomyopathy.
Pertinent laboratory results revealed a troponin of 7 ng/ml, CBC of 14,000, Hg 8.8 and creatinine of 2.8. The patient's stool was heme positive. Blood cultures were negative. Initial urine specific gravity was 10.30.
The patient received 2 units of packed red blood cells. Medical therapy included low dose B-blocker and angiotensin converting enzyme inhibitor. Her symptoms all resolved on the third day of after hospitalization. The working diagnoses were resolving viral gastroenteritis and reverse Takotsubo cardiomyopathy. Upon discharge the plan was follow up in four weeks for repeat echocardiogram, blood work, nuclear stress testing and a plan for colonoscopy.
Three weeks after discharge the patient presented with weakness, hypotension (80/50 mmHg) and melanotic stools. She was in atrial fibrillation with a rapid ventricular response of 125 bpm. She was given two liters of normal saline and spontaneously converted to normal sinus rhythm and blood pressure rose to 110 mmHg.
Physical examination revealed normal blood pressure and heart rate. Jugular venous pressure was flat and carotid upstroke was low pulse volume. Auscultation revealed a 2/6 systolic ejection murmur and a gallop. Lungs were clear and there was no peripheral edema.
ECG revealed sinus rhythm and non-specific ST changes (Figure 3). The echocardiogram revealed an akinetic apex and dynamic basal function (Figure 4 and Movie 2).
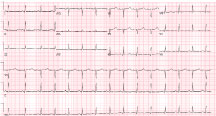 Figure 3: Sinus rhythm with non-specific ST changes.
View Figure 3
Figure 3: Sinus rhythm with non-specific ST changes.
View Figure 3
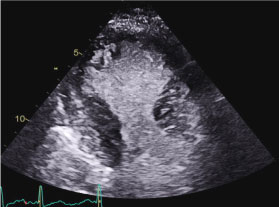 Figure 4: Apical 2 chamber end-systolic still frame revealing apical akinesia and basal contraction.
View Figure 4
Figure 4: Apical 2 chamber end-systolic still frame revealing apical akinesia and basal contraction.
View Figure 4
Movie 2: Echocardiographic Apical 2 chamber view of the left ventricle with contrast revealing left ventricular apical dyssynergy (red arrow) and left ventricular basal hyperkinesia (yellow arrow). The echocardiographic features represent the subtype of stress-induced cardiomyopathy called apical Takotsubo cardiomyopathy.
Laboratory finding revealed troponin was 2 ng/ml, Hg 9.7, WBC 4.4 and creatinine 1.1. Urine specific gravity was 10.20.
The patient was treated with empiric antibiotics. On day four of the hospitalization she stabilized and was transferred to a tertiary care center. Evaluation at the tertiary care center revealed normalization of LV function by echocardiography and normal coronary arteries at heart catheterization. Colonoscopy revealed no source for bleeding. Repeat CBC and metabolic panel were normal. Final diagnosis was viral gastroenteritis and recurring stress induced cardiomyopathy.
This case is unique with the patient having recurrent stress-induced cardiomyopathy with fluctuating regional location of the LV dyssynergy in a short interval of time. Several anatomical variants of stress-induced cardiomyopathy (SIC) have been described and four major types can be delineated based upon the distribution of regional wall motion abnormalities. The most common SIC type and widely recognized form is the apical ballooning type. Over the last decade atypical types of SIC have been increasing recognized and include the midventricular variant, basal variant and focal variant [1].
The pathophysiologic mechanisms underlying the transient left ventricular dyssynergy remain enigmatic. Most current hypotheses are based upon catecholamine surges in the setting of acute emotional or physical stress leading to catecholamine-related toxic effects. Recently data suggests direct administration of catecholamines or medications causing catecholamine surge is frequently associated with Takotsubo cardiomyopathy. Additionally chemotherapy can be a culprit [1].
Alternative proposed explanations include vasospasm of the epicardial arteries, and coronary microvascular blood flow abnormalities, but all have been disputed [2].
This case is rare in that the patient has recurrent stress induced cardiomyopathy with a different pattern of ventricular involvement within a three-week period. This patient has a sigmoid septum and there is subset of patient with sigmoid septum that has been shown to have the potential to develop a dynamic mid - cavity obstruction with dobutamine infusion or when they become dehydrated [3]. These patients can develop gradients, but most likely do not progress to a cardiomyopathy because they do not experience the high levels of catecholamines needed to induce subendocardial ischemia [4].
This patient had a predisposition, with her sigmoid septum, to develop dynamic mid-cavity obstruction forming two chambers in the LV cavity. She presented with dehydration and catecholamine stress from her systemic illness. This constellation of LV morphology and her systemic illness may have induced a gradient between the two chambers. Her normal epicardial arteries protected against subendocardial, or full thickness infarction in the high-pressure chamber.
The variable regional LV involvement may be related to the separation of the LV cavity into two chambers with the mid-cavity dynamic obstruction [2]. In reverse Takotsubo the base of the LV is the high-pressure chamber with high wall stress and the apical chamber has normal pressure and wall stress resulting the observed myocardial contraction abnormality. Potential variation in the active catecholamine receptors may potentiate the discrepancy in subendocardial blood flow between the chambers. In the apical phenotype of SIC the base of the LV is the low-pressure chamber with low wall stress and the apical chamber has high pressure and wall stress resulting the observed myocardial contraction abnormality of apical akinesis.
This variable LV morphological response to the stress of illness and hypovolemia in this patient suggests that the pathophysiology of the various types of SIC is multifactorial and that LV morphology, hypovolemia, catecholamine excess and normal epicardial coronary arteries may in conjunction determine the phenotypic expression of the SIC.
The author declares there are no conflicts of interest regarding the publications of this paper.