Hepatic angiosarcoma, Liver cirrhosis, Liver failure, Cirrhosis decompensation
Hepatic angiosarcoma (HAS) is a rare malignancy that accounts for 2% of all hepatic primary tumors [1]. It has been related to exposure to carcinogens (vinyl chloride, radiocontrast material, thorium dioxide, androgenic steroid, arsenic...) [1,2]. The diagnosis is challenging due to the lack of specificity of the symptoms and the heterogeneous appearance on radiologic images, especially if the patient does not have previous history of occupational exposure. Its prognosis is poor [1,3].
A 64-year-old male with heavy alcohol consumption, previous spontaneously resolved HBV infection but no occupational exposure risk factors, was admitted to hospital for hematemesis. Blood tests on admission were as follows: hemoglobin 15.3 g/dL (13.5-18); mean cell volume (MCV) 101 fL (78-100); white cell count (WCC) 9840/μL (4000-10,000); platelet count 51,000/μL (150,000-400,000); international normalized ratio (INR) 1.44; bilirubin 2.91 mg/dL (0.2-1.0); albumin 2.4 g/dL (3.2-4.8); aspartate aminotransferase (AST) 76 U/L (10-34), alanine transaminase (ALT) 51 U/L (10-44) and γ-glutamyl transpeptidase (GGT) 254 U/L (11-50) .
Urgent upper gastrointestinal endoscopy showed bleeding from esophageal varices, which was successfully controlled with rubber-band ligation. Somatostatin was administered for 5 days. An abdominal ultrasound examination showed an enlarged and slightly lobulated liver, a dilated portal vein and multiple nonspecific hepatic nodules. Multiphase computerized tomography (CT) and, secondly, a magnetic resonance (MRI) imaging revealed multifocal and isodense masses in precontrast images. The majority of them were hypovascular. However, in some lesions, peripheral enhancement was seen in the arterial phase, but not in portal and delayed phases. There was no definite washout (Figure 1). Due to these findings, inconsistent with hepatocellular carcinoma, the patient underwent percutaneous liver biopsy, revealing a diffuse infiltration by a vascular malignancy with a high degree of atypia and cellular pleomorphism (Figure 2A). Immunohistochemically, the tumor cells expressed CD31, CD34 (Figure 2B), Factor VIII (Figure 2C) and vimentin Ki-67 index was 30%. A thoracic CT ruled out the presence of metastasis, while a positron emission computed tomography (PET-CT) revealed multiple bone infiltration.
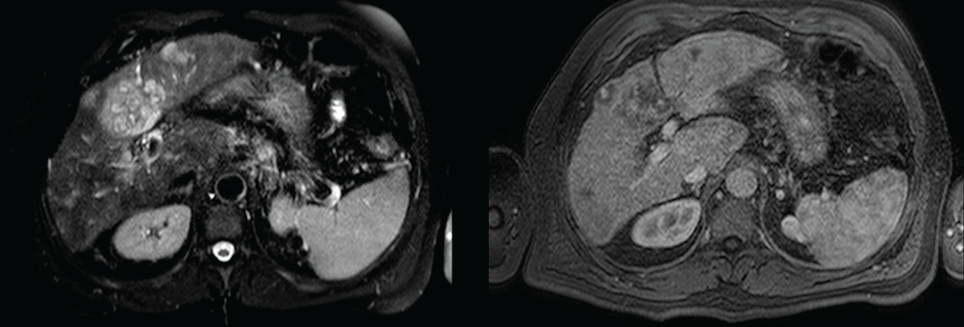 Figure 1: MRI showing nonspecific hepatic nodules.
View Figure 1
Figure 1: MRI showing nonspecific hepatic nodules.
View Figure 1
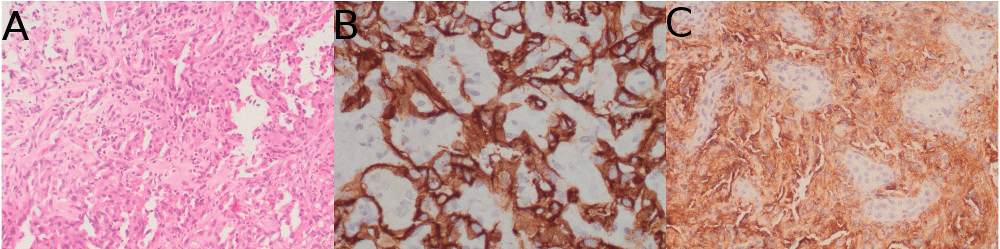 Figure 2: A) hematoxilin-eosin; B) Inmunohistochemical staining: CD34; C) Factor VIII.
View Figure 2
Figure 2: A) hematoxilin-eosin; B) Inmunohistochemical staining: CD34; C) Factor VIII.
View Figure 2
Due to the extension of the tumor and the presence of thrombocytopenia, targeted therapy with Pazopanib was started. Two weeks later, the patient was readmitted for severe encephalopathy and a posterior reversible encephalopathy syndrome (PRES) was suspected. Brain MRI confirmed the diagnosis and, finally the patient passed away three days later.
HAS is a rare entity but nonetheless represents the most frequent mesenchymal malignancy of the liver [1,2]. It originates from liver vascular endothelial cells and lymphatic vessels cells [1]. Around 200 cases are diagnosed annually worldwide. It is more likely to affect those between 60 and 80-years-old, with a male-to-female ratio of 3:1.
HAS is associated with exposure to chemical carcinogens, including vinyl chloride, radiocontrast material, thorium dioxide, androgenic steroid and arsenic [1,2,4].
Vinyl Chloride-induced KRAS and p53 mutations have been documented [5], albeit the exact mechanisms of chemical-related tumorigenesis have not been fully elucidated yet. It has also been associated to vascular diseases such as Neurofibromatosis type I [6]. Despite of all the risk factors described, in most cases no etiological factors are found, making the diagnosis a challenge.
Reports regarding the association of HAS with cirrhosis have thrown contradictory findings. Some studies suggest the comorbidity of the two of them, but whether playing a direct causative role or just coexisting is not clear [2,4]. Others, argue against the association. Data from a study carried out in Taiwan [4], which is an endemic area of viral hepatitis B, does not support HBV infection or HBV-cirrhosis being a risk factor for HAS. Locker, et al. [7] reviewed 103 cases of HAS. Among those (60 cases) with no definite exposure to chemicals, only 3 were found to have liver disease. On the other hand, Pickhardt, et al. [8] reported 15 cases (42.9%) of liver cirrhosis among 35 HAS cases and a positive association with liver cirrhosis was also found by Soini, et al. [5]. Similary to the case we present here, in some of these reports patients present alcoholic liver cirrhosis; however, to the date, alcohol consumption has not been demonstrated to be a carcinogen related to the appearance of hepatic angiosarcoma.
Symptoms related to HAS are nonspecific. Abdominal pain, weakness, fatigue and weight loss are frequent. The most common clinical findings are hepatosplenomegaly, ascites, jaundice and anemia. Due to his rapid growth, it has been related to acute liver failure [9] and cirrhosis decompensation. Spontaneous rupture of liver angiosarcoma is reported in 15-27% of cases.
The diagnosis requires careful assessment of cross-sectional imaging findings and histological evaluation [8]. Common imaging findings of primary HAS include rapidly progressive multifocal tumors and hypervascular foci on the late arterial phase. Large hypovascular regions are common. Interestingly, tumor enhancement pattern may resemble cavernous hemangiomas and lead to misdiagnosis. In cirrhotic livers, lack of tumor washout of hypervascular lesions argues against hepatocellular carcinoma and makes histopathological analysis mandatory.
Histopathology may show different patterns of vascular channels and dilated sinusoidal or cavernous spaces. Immunohistochemical staining is useful for confirmation, usually expressing CD34, CD31 and factor VIII-related antigen [7]. Differential diagnosis must consider other entities such as hemorrhagic hepatocellular carcinoma or hepatic peliosis. In up to 35% of cases, the diagnosis is reached during autopsy.
Survival rates are poor. Zheng, et al. described median survival of 5 months [3]. Standard treatment is surgical resection, although is only feasible in less than 20% of cases. Liver transplant is controversial due to high recurrence rate. Patients with metastatic angiosarcoma are, if tolerated, treated with chemotherapy. However, no regimens have been established and several agents may be used [3,10].
In this case, no chemical exposure was found. At diagnosis, targeted therapy with Pazopanib was considered as first-line treatment due to the presence of metastasis and thrombocytopenia. The patient had not presented previous cirrhosis-related decompensations. The rapid growth of the tumor might have been the trigger for the variceal bleeding. Liver cirrhosis may play a role in the development of HAS. However, there is not enough evidence to support this association yet. Through a multicenter joint effort, a most accurate characterization of this rare entity may be possible, therefore allowing to clarify the association between cirrhosis and HAS.