Atypical atrial tachycardia, Catheter ablation, Sudden termination
Careful attention should be paid to the area behind the Eustachian ridge, if an unexpected sudden termination of an AT occurs after a prior cavo-tricuspid isthmus ablation.
A 56-year-old woman without any significant past illnesses was suffering from frequent palpitations due to drug refractory paroxysmal atrial fibrillation (AF). The findings from the chest X-ray, laboratory data including the thyroid hormone level, and echocardiography data including the left atrial diameter were within normal limits. Catheter ablation was performed in other hospitals, however, 5 ablation sessions were required to cure the AF in this case.
An extensive pulmonary vein isolation (PVI) and additional cavotricuspid isthmus ablation was successfully performed. Atrial premature beats were reproducibly determined from left superior and inferior PVs, and AF was followed by left inferior PV triggers. After PVI, atrial burst pacing failed to induce AF.
A PVI for the reconnected PVs and an additional left atrial roof line was successfully created. A drug refractory atrial tachycardia (AT) occurred 9 months after the ablation. Atrial flutter was not induced during PVI procedure in 1st and 2nd sessions. The additional tricuspid isthmus line between tricuspid valve and inferior vena cava (IVC) was created, and the presence of atrial double potentials was finally confirmed.
In the 3rd session, an AT with the earliest activation at the coronary sinus (CS) ostium was easily induced. During the mapping procedure in the right atrium, a sudden AT termination without the following inducibility (bump) occurred. In the 4th session, the Ensite array was used to assess the circuit of the AT. During the insertion of the array into the right ventricular outflow tract, the AT suddenly terminated. After the abrupt termination, the AT could no longer be induced in either session.
Even after the 4 sessions, a drug refractory AT frequently occurred. An intravenous antiarrhythmic administration (Pilsicainide hydrochloride; 50 mg, Disopyramide Phosphate; 50 mg, Cibenzoline Succinate; 70 mg) also was not effective, and multiple direct cardioversions of more than 40 times were required to terminate the AT. This patient was referred to our hospital to undergo the 5th ablation session for the AT.
Four electrode catheters were inserted via the right femoral and cervical veins, and positioned in the coronary sinus (6Fr 20 pole catheter), high right atrium (5Fr quadripolar catheter), right ventricular apex (5Fr quadripolar catheter), and His bundle region (5Fr 10 pole catheter). The intracardiac electrograms and surface electrocardiography (ECGs) were continuously monitored and recorded using an EP Workmate (St. Jude Medical, Minneapolis, Minnesota, USA).
No retrograde ventricular-atrium (VA) conduction was observed. An AT (cycle length; 500 ms) (Figure 1a) was easily induced by an atrial extrastimulus and/or burst pacing from the right atrium. Careful 3D mapping revealed the activation sequence of the AT was from the CS ostium, to the septum, then the anterior wall, and to the lateral wall in the right atrium (Figure 1b). In the right atrium, the earliest activation site was recorded around the CS ostium, which coincided with the onset of the P wave on the 12 lead ECG. Concealed entrainment was obtained at the CS ostium and right lateral wall (Figure 2). Double potentials were observed at the cavotricuspid isthmus, however the AT suddenly terminated during the mapping at the proximal part of the tricuspid isthmus and the AT could no longer be induced (Figure 3a). During sinus rhythm, continuous fragmented conduction, possibly related to the maintenance of the AT, was recorded at the junction of the inferior vena cava (IVC) ostium and Eustachian ridge (Figure 3b). RF energy delivered with a irrigated 4 mm tip catheter (35 W, maximal temperature of 41 degree) was applied to the site exhibiting the fragmented conduction. After ablation, we confirmed During the follow-up period, no further tachyarrhythmias were observed after this session.
The documented tachyarrhythmia was firstly suspected to be a focal AT, however we finally realized the course of the AT was consistent with that of a counter clockwise rotating cavo-tricuspid isthmus dependent atrial flutter (typical flutter). Typical flutter generally demonstrates an entire atrial activation in the extremity leads, and occupies the entire activation of both atria. The 12 lead ECG of typical flutter usually has negative flutter waves in leads II and III and positive atrial deflections in lead V1, and the cycle length is nearly 170 to 240 ms [1]. In this case, the P wave polarity during the AT of the surface electrogram was unlike that of typical flutter. The AT cycle length significantly prolonged up to 500 ms, and a remarkable conduction delay was caused by the cavo-tricuspid isthmus region. The ECG characteristics seemed to vary after the prior AF ablation. An extensive pulmonary vein ablation may alter the normal activation pattern of the left atrium, and typical flutter may not appear as a typical feature of the surface electrogram [2]. These atypical 12 lead ECG features of common flutter may be incorrectly diagnosed as another type of atrial tachyarrhythmia.
In this case, the method for assessing the conduction along cavo-tricuspid isthmus might be insufficient to confirm the bidirectional conduction block in the prior session. Presence of concrete double potential through the entire ablation line between tricuspid valve and IVC should be confirmed under the pacing from both septal and latera sides of the created line, and the activation detour should be also assessed by a multipolar catheter located along tricuspid valve. In addition, differential pacing maneuvers can be helpful to determine the presence of complete block line cavo-tricudpid line, and the sensitivity and specificity for linear block were 100% and 75%, respectively [3]. However, these parameters may be confounding and the further careful attention should be paid in the cases with the remarkable prolonged conduction delay.
The structure of the cavo-tricuspid isthmus region is variable and distinct, but the Eustachian ridge consists of a major muscular part of typical atrial flutter in the majority of cases [4]. Depending on a complex thick musculature or deep recess, it is sometimes difficult to keep the catheter stable without falling into the IVC. Previous studies suggested that a higher number of applications is likely to be required at the region of the Eustachian ridge, and that area behind the Eustachian valve is curucial for success in achieving complete block in the cavo-trisupid isthmus [5]. In this case, a remarkable prolonged conduction serving as a crucial substrate for the clinical typical flutter was observed behind the Eustachian ridge, and was probably due to the prior tricuspid isthmus ablation. Incomplete conduction block can be overlooked in prior sessions, because it is sometimes difficult to confirm the complete block at the cavo-tricuspid isthmus by differential pacing in cases with a long conduction time in that region [6].
An unexpected sudden bump repeatedly occurred during the catheter manipulation and/or Ensite array insertion in spite of careful mapping. Sudden bump is unlikely to be caused by the contact of the distal tip of the ablation catheter, because the double potentials reflecting an existent local conduction block were clearly recorded by the ablation catheter electrodes at the bump site in the 4th session (Figure 3). Continuous fragmented local electrograms were recorded at a further proximal site behind the Eustachian ridge; therefore the direct mechanical contact with that region may have lead to the unexpected sudden bump phenomenon. The fluoroscopic view (Figure 3) suggested that contact of the body of the catheter and/or a long sheath seems to have easily occurred in that area. For that reason, careful attention should be paid to the area behind the Eustachian ridge as a favorable bump site, if an unexpected sudden bump of an AT occurs after a prior cavo-tricuspid isthmus ablation.
Naoto Kino, MD: Data collection and analysis;
Toshiya Kurotobi, MD PhD: Data collection and analysis, manuscript writing;
Kazato Ito, MD: Data collection and analysis;
Daisuke Tonomura, MD: Data collection and analysis;
Kentaro Yano, MD: Data collection and analysis;
Chiharu Tanaka, MD: Data collection and analysis;
Yoshihisa Shimada, MD, PhD: Data collection and analysis.
None.
None.
There are no relationships with the industry or conflicts to disclose for this study. All authors agreed to the submission of this paper.
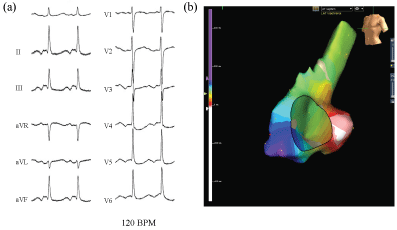
Figure 1: The surface electrocardiogram during an atrial tachycardia (AT) (a); The P wave polarity during the AT was biphasic in leads II, III, and aVF, and flat in leads I and V1. The AT cycle length was 500 ms. The course of the AT without covering the entire cycle length can be visualized from the CS ostium to the LRA (b) (arrow; red→yellow→green→blue→purple). LRA: Lateral right atrium.
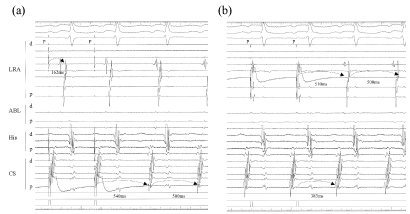
Figure 2: Concealed entrainment of the AT was achieved from the CS ostium and LRA (star mark; pacing site). The atrial activation sequence was almost the same as that of the AT, and a post pacing interval with the same atrial sequence was consistent with the cycle length of the AT at each site (CS ositum; + 10 ms, lateral RA; + 40 ms). The conduction time from the CS ostium to the RA lateral was 162 ms, and that of LRA to the CS ostium was 389 ms; which exhibited a long conduction time at the cavotricuspid isthmus lesion covering approximately 80% of the AT cycle length.
LRA: Lateral right atrium; HIS: His bundle; ABL: Ablation site; dis: distal electrode; prox: proximal electrode.
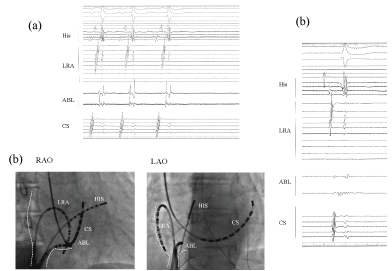
Figure 3: The local electrogram at the proximal cavotricuspid isthmus representing a sudden termination of the AT. Double potentials were recorded by the ablation catheter. While drawing the catheter back toward the IVC ostium, the AT suddenly terminated with no further AT inducibility (a); In this fluoroscopic view, the ablation catheter at the AT bump site is located at the junction at the Eustachian ridge. The site just proximal to the site of the AT bump represents continuous fragmented potentials, which implies a remarkable slow conduction of the AT circuit (b). White dot; ostium of inferior vena cava; IVS: Inferior vena cava.